Real-world Experience with Remote Electrical Neuromodulation in the Acute Treatment of Migraine
Stewart J. Tepper MD et al, Pain Medicine, 2020
Abstract
Objective: Remote electrical neuromodulation (REN) is a nonpharmacological acute migraine treatment that stimulates upper-arm peripheral nerves. The aim of this investigation was to evaluate the effectiveness and safety of REN for acute treatment of migraine in a real-world setting.
Methods: Real-world data were collected from patients who were using REN (Nerivio®, Theranica Bio-Electronics Ltd., Israel) between October 1, 2019, and March 31, 2020. Patients recorded their symptoms at baseline, two hours, and 24 hours post-treatment. Patients were stratified based on the type of visit and provider; in-person visits with headache specialists (HS group) or virtual visits with nonheadache specialists (NHS group). Efficacy outcome focused on intra-individual consistency of response across multiple attacks.
Results: We found that 58.9% (662/1,123) of the patients in the HS group and 74.2% (23/31) of the patients in the NHS group experienced pain relief at two hours in at least 50% of their treated attacks and 20.0% (268/1,339) of the patients in the HS group and 35.6% (16/45) of the patients in the NHS group experienced pain freedom at two hours in at least 50% of their treated attacks. The effects of REN on associated symptoms and improvement in function were also consistent in both groups. The incidence of device-related adverse events was very low (0.5%).
Conclusions: Real-world data confirm that REN results in meaningful clinical benefits with minimal side effects. REN may provide an effective drug-free treatment option for achieving consistent relief from migraine symptoms and may reduce the use of acute medications.
Introduction
Migraine is a disabling neurological condition that affects 12% of the general population. It is characterized by recurrent, disabling headache attacks often as sociated with nausea, vomiting, photophobia, and phonophobia. The symptoms of a migraine attack are typically managed with acute pharmacological care that includes nonsteroidal anti-inflammatory drugs (NSAIDs) and simple analgesics such as acetaminophen or aspirin, which may be combined with caffeine, opiates, or with specific migraine treatments such as triptans, ergots, lasmiditan, and gepants. However, these treatments may be ineffective, poorly tolerated, contraindicated, and if used in excess, may lead to medication overuse headache (MOH) and migraine chronification.
Noninvasive neuromodulation represents an emerging alternative for the acute treatment of migraine. Remote electrical neuromodulation (REN) is a novel drug-free acute treatment of migraine, in which upper-arm peripheral nerves are stimulated to induce conditioned pain modulation (CPM), a descending endogenous analgesic mechanism in which subthreshold conditioning stimulation inhibits pain in remote body regions. The REN device is a wireless, wearable, battery-operated stimulation unit controlled by a smartphone software application. The device is applied for 45 minutes to the lateral upper arm between the bellies of the lateral deltoid and the triceps, so that it will mainly stimulate small skin nerves.
The safety and efficacy of REN have been confirmed in two randomized controlled trials, demonstrating significant clinical benefit vs placebo sham with low incidence of adverse events (AEs) and good tolerability. Specifically, in a randomized, double-blind, placebo sham-controlled clinical trial, REN relieved migraine pain in 66.7% and eliminated pain in 37.4% of patients within two hours after treatment. A randomized controlled trial is the gold standard of clinical research, as it is designed to eliminate bias and ensure that efficacy and safety are reliably evaluated, but it is limited by sample size, short duration, and restricted patient populations. Furthermore, the results obtained in a controlled environment may not reflect how a therapy would be used in everyday practice, where patients are not screened, monitored, or consistently provided with training and guidance.
Postmarketing surveillance is designed to assess the efficacy and safety in larger and more diverse populations and in various real-world environments and situations. As a digital therapeutic device (i.e., electroceutical), the REN device enables prospective collection of electronic patient-reported outcomes in real-world clinical practice. We investigated evidence regarding clinical benefits and safety following the use of REN during the first six months in which the device was available in the United States. Our data set included two cohorts of different migraine populations. One cohort included patients who regularly visit a health care provider (in-person visit) for their headaches, which in the current sample mainly included headache specialists ( 85%). This population is characterized by more frequent migraine attacks (i.e., chronic migraine), increased likelihood to experience severe pain intensity, high functional disability, less robust response to triptans, and high discontinuation rates of prescribed acute treatment due to lack of efficacy. The second cohort includes patients who were managed through a single-specialty or multispecialty telemedicine platform in which a health care provider is connected to the patients based on the treatment they are seeking; these platforms typically include fewer or no follow-up visits. In this sample, treatments were prescribed by nonheadache specialists, mainly including physicians practicing family medicine or internal medicine, consistent with the typical characteristics of telemedicine physicians.
The analyses focused on intra-individual consistency of response across multiple attacks. Demonstrating consistency of a treatment is clinically important, especially in a real-world setting, as it would indicate that a treatment can be relied on by the patients, which can improve adherence, reduce migraine-related functional disability and anxiety, and increase overall confidence in efficacy.
Methods
Population and Data Collection
The real-world data of REN were collected from patients across the United States who were using the REN device between October 1, 2019, and March 31, 2020. Patients were stratified based on the type of visit and health care provider: in-person visits with headache specialists (HS group) or virtual visits with nonheadache specialists (NHS group).
All patients had to install the Nerivio app on their smartphones, create an account, and accept the terms of use, which specified that they are not obligated to provide personal information (i.e., they may treat without providing feedback), that providing personal information is done of their own free will, and that their de-identified data may be used for research purposes. The app includes a secured, personal migraine diary, which enables patients to record and track their migraines and other headaches. At the beginning of each treatment, patientscan record their symptoms including pain level (none, mild, moderate, severe), presence of nausea and/or vomiting, photophobia, phonophobia, and functional disability. To assess functional disability, participants recorded their response to the following question: “How do you rate your ability to perform your usual activities?” using a four-point scale (“no limitation,” “some limitation,” “moderate limitation,” “severe limitation”). Patients also have an option to record their symptoms at two hours and 24 hours after the treatment and indicate if and which medication was used.
As these data present real-world use, in contrast to clinical investigations, patients did not receive instructions or training and were not obligated to record their symptoms. Thus, the analysis population included patients who had performed at least one evaluable treatment (a treatment with pain level data at baseline and at two hours post-treatment).
The REN Device
REN is a wearable device applied to the upper arm and stimulates C and Ad noxious fibers using a modulated, symmetrical, biphasic, square pulse with a pulse width of 400 ls, modulated frequency of 100–120 Hz, and up to 40 mA output current, which can be adjusted by the patient.
Outcomes
Efficacy outcomes included pain relief at two hours (a decrease in headache pain from moderate or severe at baseline to mild or no pain at two hours after treatment), pain freedom at two hours (a decrease in headache pain from mild, moderate, or severe at baseline to no pain at two hours after treatment), improvement in function at two hours (improvement in at least one grade between baseline and two hours), return to normal function at two hours (no functional disability at two hours), disappearance of at least one associated symptom of nausea and/or vomiting, photophobia, and phonophobia at two hours (only symptoms that were present at baseline were included in this analysis), sustained pain relief at 24 hours (mild or no pain at 24 hours without medication or reuse of REN within 24 hours of treatment after pain relief was achieved at two hours), and sustained pain freedom at 24 hours (no pain at 24 hours without medication or reuse of REN within 24 hours of treatment start after pain freedom was achieved at two hours).
Within-subject consistency, defined as response in at least half of treated attacks, was calculated for each parameter.
Data Analysis
The population of REN users included all patients who used the device at least once. Presumably, the two cohorts in this data set differ in functional disability, symptomatic and functional profiles, rates of comorbidities, and response to treatments, as most patients who see headache specialists are people with chronic migraine. Thus, all analyses were conducted in each group separately.
Efficacy analyses were conducted on patients with at least one evaluable treatment. As this is real-world use, some patients combined REN treatment with an acute medication in some of their treatments. In order to assess the clinical benefit of the device and isolate it from the effect of additional treatments used for an attack, all efficacy analyses were conducted on treatments in which no medication was taken within two hours post-treatment.
A response in each associated symptom (nausea/vomiting, photophobia, phonophobia) is defined as change from presence of a specific associated symptom at baseline to absence of the same associated symptom at two hours post-treatment. Patients with presence of at least one symptom at baseline and data at two hours are included in the analyses. For improvement in function end points at two hours, all patients with baseline values of “some limitation,” “moderate limitation,” or “severe limitation” and data at two hours are included in the analyses. A response was defined as a reduction of at least one grade at two hours post-treatment.
To assess the safety of REN, all AEs were classified in relation to their severity, duration, and possible causal relationship to the device. The primary safety variable was the proportion of patients reporting one or more device-related AEs. The safety analysis was conducted on all patients who treated at least one attack with the device (pooled analysis on both cohorts).
For continuous variables, mean and standard deviation are provided. For categorical variables, the number and percentage of patients in each category are provided. Group differences were evaluated using a two-tailed chisquare test. A P value <0.05 was used to identify statistically significant effects. Data were analyzed with IBM SPSS statistics software, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
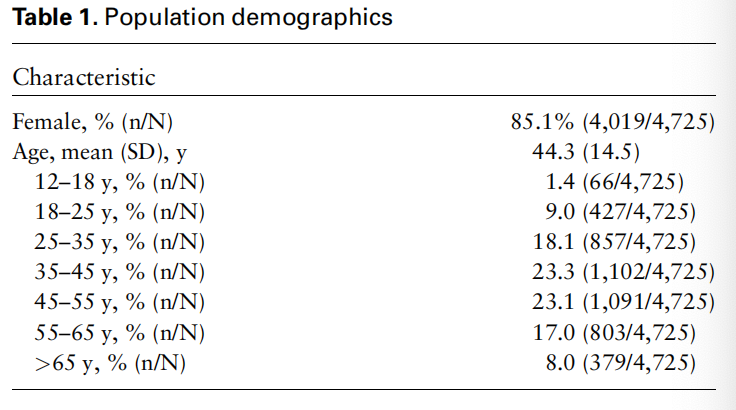
Patients and Treated Migraine Headaches Data were collected across the United States from October 1, 2019, to March 31, 2020. A total of 4,725 patients used REN during that time period (mean age ¼ 44.3 6 14.5 years, 85.1% females) (Table 1). Of these, 4,573 were prescribed through in-person visits with headache specialists (HS), and 152 were prescribed through telemedicine platforms (nonheadache specialists [NHS]). All patients performed a total of 25,984 treatments, an average of 5.5 treatments per patient.
Efficacy analyses were conducted on 1,384 patients who performed at least one evaluable treatment in which no medication was taken (total of 2,953 treatments, an average of 2.1 treatments per user). Pain levels at 24 hours were recorded in 45.8% (1,353/2,953) of these treatments. Among the 1,384 patients, 1,339 patients were included in the HS group (total of 2,875 treatments, average of 2.1 treatments per user) and 45 were included in the NHS group (total of 78 treatments, average of 1.7 treatments per user).
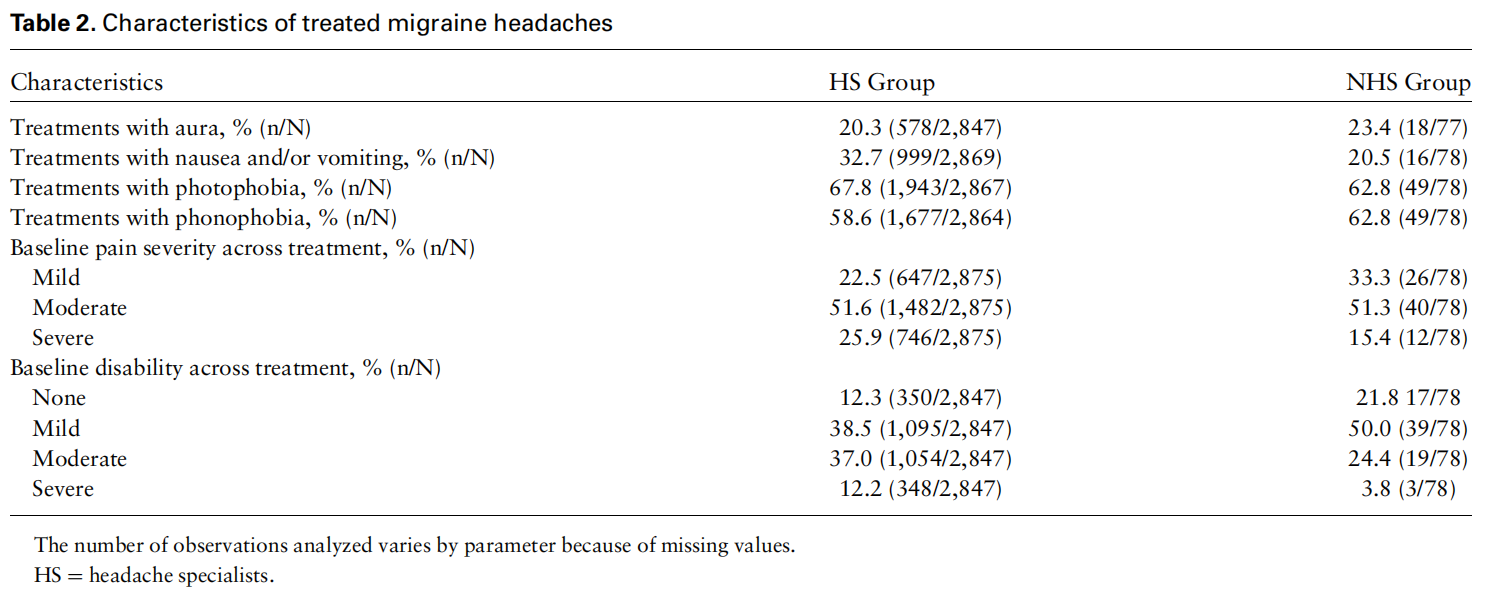
In the HS group, pain severity of treated migraine headaches was mostly moderate (51.6% [1,482/2,875]); 746/2,875 (25.9%) of the treated migraine headaches were severe, and 647/2,875 (22.5%) of the treated migraine headaches were mild. In the NHS group, pain severity of treated migraine headaches was mostly moderate (51.3% [40/78]). Twelve of 78 (15.4%) of the treated migraine headaches were severe, and 26/78 (33.3%) of the treated migraine headaches were mild. The characteristics of the treatments are depicted in Table 2.
Efficacy Outcomes
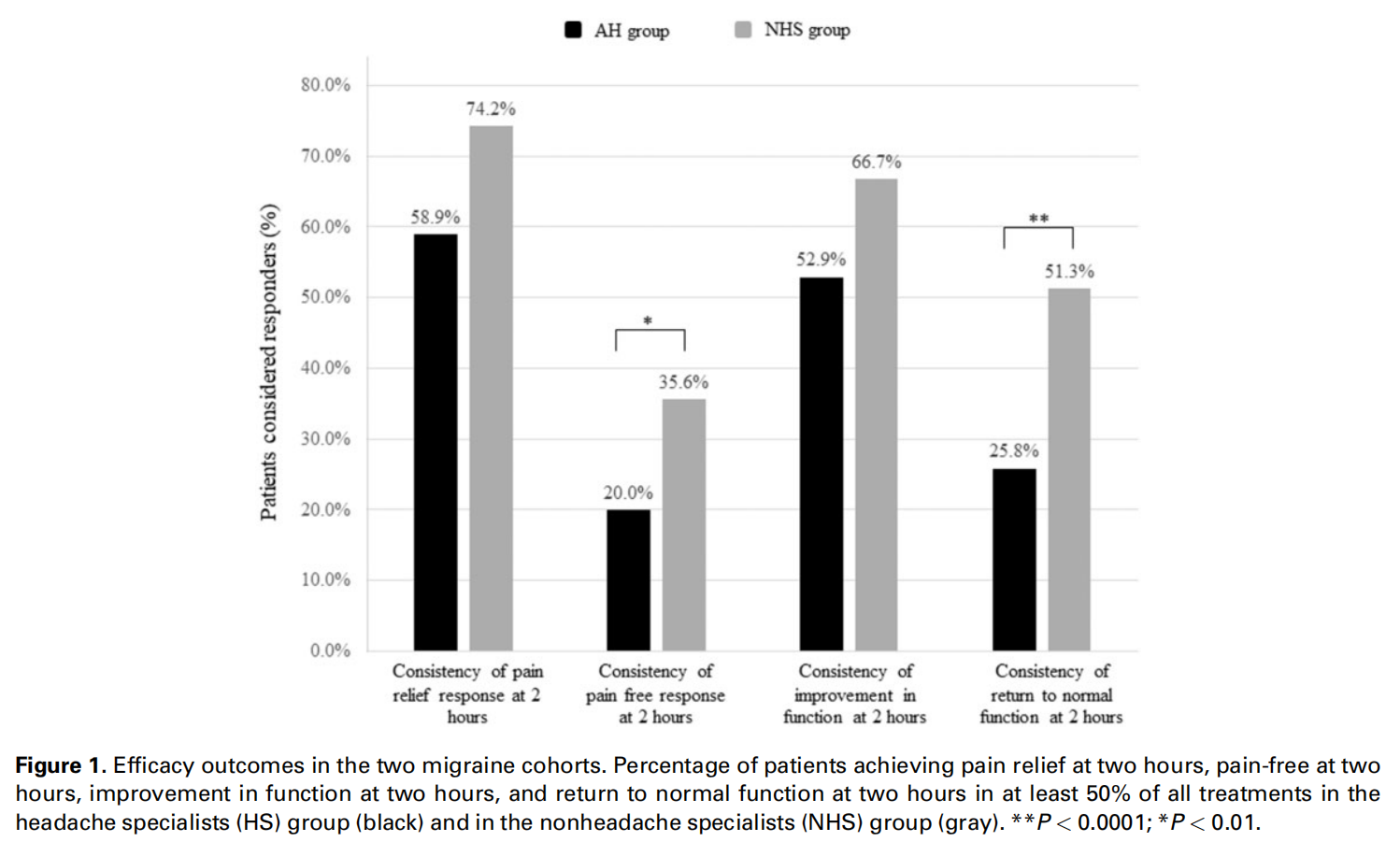
All consistency analyses were conducted on treatments in which no medication was taken at two hours posttreatment (52.7% [3,584/6,799] of all treatments in which an answer to the medication use question was provided). In the HS group, 58.9% (662/1,123) of the patients experienced pain relief at two hours in at least 50% of their treated attacks, vs 74.2% (23/31) of the patients in the NHS group (P ¼ 0.09), and 20.0% (268/1,339) of the patients experienced pain freedom at two hours in at least 50% of their treated attacks, vs 35.6% (16/45) of the patients in the NHS group (P ¼ 0.01) (Figure 1, Table 3). Furthermore, in the HS group, 55.5% (614/1,107) of the patients experienced disappearance of at least one of the associated symptoms in at least 50% of their treated attacks vs 65.8% (25/38) of patients in the NHS group (P ¼ 0.21); 52.9% (644/1,218) and 25.8% (314/1,218) of patients reported improvement in function and return to normal function at two hours in at least 50% of their treated attacks in the HS group vs 66.7% (26/39) and 51.3% (20/39) in the NHS, respectively (improvement in function: P ¼ 0.09; return to normal function: P ¼ 0.0001) (Figure 1).
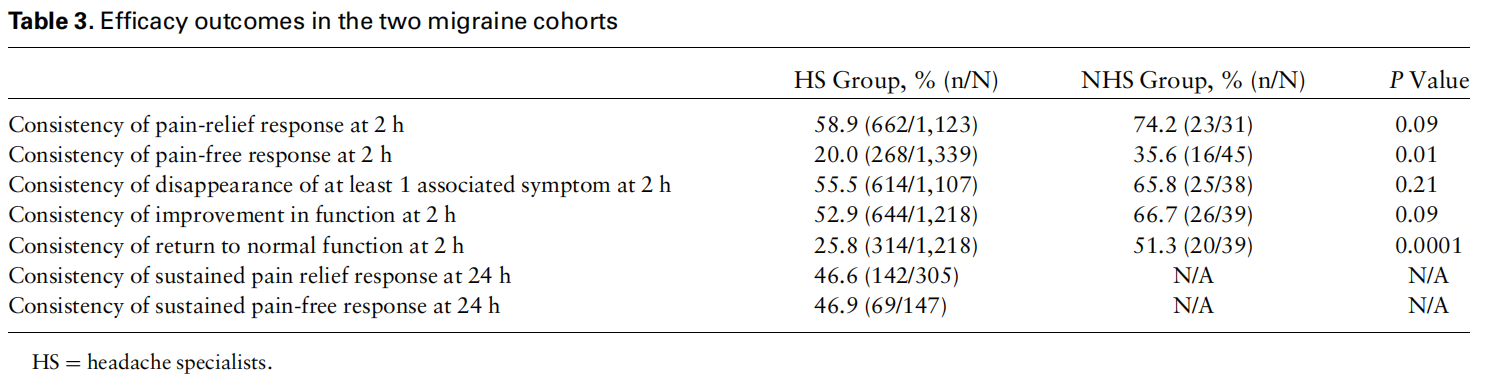
In the HS group, 46.6% (142/305) and 46.9% (69/ 147) of patients reported sustained pain relief and sustained pain freedom at 24 hours in at least 50% of their treated attacks, respectively. The sample size was too small (N < 15) to analyze sustained pain response at 24 hours in the NHS group.
Safety
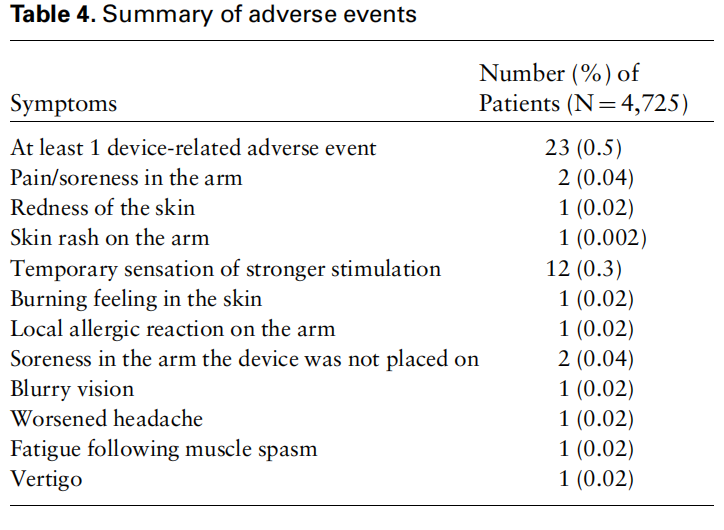
Safety analyses were performed on all 4,725 patients who used the device at least once (total of 25,984 treatments). Adverse events could be reported by patients, caregivers, and health care professionals through customer support telephone hotlines and e-mail.
There were 23 patients (0.5%) who experienced at least one device-related adverse event (total of 24 adverse events) (Table 4). Expected device-related AEs included pain/soreness in the arm (two patients; in one case, it occurred following the use of the device for four consecutive days and required ibuprofen to resolve), redness of the skin (one patient; the redness lasted for several days), and skin rash on the arm (one patient). Twelve patients reported an unexpected device-related adverse event in which a temporary sensation of stronger stimulation was experienced. This adverse event was categorized as mild, resolved shortly after the treatment, and did not require medical attention. The AE was investigated and deemed to be due to a device malfunction, which was fixed. Following this correction, there were no additional reports of this type of AE. Additional unexpected device-related AEs included burning feeling in the skin (one patient), allergic reaction (one patient), soreness in the arm the device was not placed on (two patients; in one case the soreness resolved as soon as the treatment ended, and in one case it resolved after 48 hours), blurry vision (one patient), worsened headache (one patient), fatigue following muscle spasm (one patient), and vertigo (one patient, although this was deemed to be only possibly related to the device). All AEs were categorized as not serious and resolved within 24 hours of treatment.
Discussion
This real-world analysis demonstrates that REN offers an efficacious treatment option with a favorable safety profile. Acute treatment of migraine attacks resulted in clinically meaningful benefits in at least 50% of treated attacks in two cohorts of migraine patients, patients with high frequency of headaches and increased disability who regularly visit headache specialists, and patients who consulted with a nonheadache specialist via a virtual visit, with characteristics more similar to the general migraine population. Overall, pain relief and pain-free rates at two hours in a naturalistic setting were similar to those reported in randomized controlled trials, studies in which REN was compared with usual migraine therapy, and open-label extension studies. This indicates that the levels of efficacy reported in controlled clinical trials can been reproduced in routine practice.
People with migraine experience numerous migraine headache days per month, which requires frequent use of acute medications. Consistent efficacy and tolerability over multiple migraine attacks are, thus, important attributes of acute therapy and have accordingly been identified by patients as a desired aspect of acute treatments for migraine. Collecting real-world data in real time using a system integrated in the app enabled evaluation of within-subject consistency of response across numerous attacks.
These real-world data consisted of two migraine cohorts that differed in the type of visit and the health care provider they see, patients who regularly attend inperson visits with headache specialists (HS group) and patients undergoing virtual visits with nonheadache specialists (NHS group). Most patients in this postmarketing surveillance were in the HS group, mostly representing the chronic migraine population, which includes more severely disabled patients. This is supported by the high proportion of attacks with severe pain at baseline and higher rates of moderate to severe migraine-related functional disability at baseline, compared with the NHS group. The characteristics of the NHS group, on the other hand, represent the general migraine population and the population included in acute treatment studies, which excludes chronic migraine patients.
Overall, the data demonstrate consistent efficacy, with no significant reduction in therapeutic benefits across treatments. Importantly, the pain responses at two hours were sustained at 24 hours, which accords with some of the most common patient expectations for acute migraine therapy, namely long-lasting pain relief and low rate of migraine recurrence. Notably, the clinical benefits of REN in a sample that represents the broad migraine population are higher, with >70% of the patients in the NHS group achieving pain relief in more than half of their attacks and >35% achieving pain freedom in most attacks. Another significant difference between the two cohorts was demonstrated in the return to normal function at two hours. The difference between the two cohorts in pain freedom and return to normal functioning at two hours may stem from the higher rates of severe headaches and moderate to severe disability at baseline in the HS group. Alternatively, it is possible that REN was one of the first treatments that patients in the NHS group had tried, which may have resulted in higher placebo response rates, which could account for some of the greater treatment response in this group. It is important to note that the sample size of the NHS group is small; thus further studies are warranted to assess this hypothesis.
The efficacy of REN is superior to that reported for other neuromodulation devices intended for acute migraine treatment, with within-subject consistency of pain relief at two hours of 59–74% compared with 47% of noninvasive vagus nerve stimulation (nVNS). Our findings also suggest that REN is equivalent to triptans, which have a 47–72% consistent pain relief response rate and a 14–42% consistent pain-free response at two hours. In addition, consistent sustained pain-free response between two and 24 hours was achieved by 47% of patients with REN compared with 35% of patients using sumatriptan/naproxen sodium. Furthermore, the consistency of elimination of functional disability response in this investigation is similar to that reported for some rizatriptan. The limitations of these comparisons are that they are not a head-to-head trial with REN, that the design, number of subjects, and number of treatments vary between studies, and that migraine populations may differ.
Our findings also demonstrated that REN eliminates the associated symptoms of migraine nausea, photophobia, and phonophobia. In clinical trials, the most bothersome symptom (MBS) of these three symptoms is an important primary end point. As in this naturalistic setting patients did not necessarily identify a single MBS, we measured response in at least one of the associated symptoms present at baseline for each attack. This approach corresponds to recent clinical trials in which MBS was determined immediately before taking the study medication. Although this analysis included response in an associated symptom that may not be considered most bothersome, it supports that treating with REN results in disappearance of at least some of the associated symptoms. This approach may be more adequate when measuring consistent efficacy across multiple attacks, as it controls for intra-individual differences in MBS between attacks. Improvement in function at two hours was also consistent across attacks, with 53–67% of patients achieving this outcome. Between 26% and 51% of patients returned to normal function at two hours in most of their attacks, similar to results reported with lasmiditan, ubrogepant, and rimegepant, although these were reported for a single attack.
The current data also support the willingness of patients to adopt a drug-free treatment. In 52.7% of all treatments, medication was not taken at two hours posttreatment, suggesting that the device may reduce reliance on medication, and consequently may reduce the risk of developing medication overuse headache. Future postmarketing surveillance analyses focusing on medication use patterns should be conducted to provide more detailed insights on medication use among REN users. As this is one of the largest real-world data sets among migraine patients, future investigations can also provide insights on how and when people with migraine use acute medication to treat their attacks.
Real-world experience with REN also shows that this treatment is well tolerated and safe. The low rate of device-related adverse events (0.5% of 4,725 patients) and the lack of device-related serious adverse events point to a favorable safety profile, especially compared with the reported rates of current acute treatments such as triptans, other neuromodulation devices, and new acute treatments including lasmiditan, ubrogepant, and rimegepant.
This investigation provides real-world evidence from prospective data in clinical practice without the limitation of postmarketing surveillance studies (e.g. patient and physician bias and less rigorous outcomes). Realworld efficacy data are often collected via retrospective evaluations (questionnaires), which are limited by patients’ recollection abilities or by prospective naturalistic-designed clinical studies, which may still introduce selection bias. The mobile application of the REN device, in which patients can record their symptoms in real time, provided the opportunity to collect prospective efficacy data similarly to the way these data are collected in controlled clinical studies, but in real time. However, as we rely on voluntary reporting, our results may have been biased, with less satisfied patients tending to record their symptoms in more treatments—or vice versa, that satisfied patients more likely to record their symptoms. However, our findings are similar to those observed in controlled clinical studies, in which all patients are instructed to record all of their symptoms. Relying on voluntary reporting reduced the number of treatments available for analyses, as only 26% of the 26,000 treatments symptoms at two hours were recorded. Nevertheless, the migraine diary as an integral part of the REN system enabled production of a large data set, similar to that obtained in retrospective postmarketing (phase 4) studies of drugs . Further analyses should be conducted to assess efficacy in a greater average of treated attacks per patient during a longer period, which will also enable investigation of long-term acute use.
There are additional limitations to these data. First, the efficacy results are not placebo controlled. However, even if accounting for the placebo response rate of 45% observed for two-hour pain relief consistency in previous studies of REN or 32% of other devices for acute treatment, the therapeutic gain in this study ( 27% in the HS group, 42% in the NHS group, and 27% in the entire study population) remains clinically meaningful. Another drawback to interpreting the data is the lack of an International Classification of Headache Disorders, 3rd Edition, diagnosis on these patients. However, most of the patients in this analysis were prescribed by US headache specialists working in headache clinics; thus the presumption is that these patients had a mix of migraine with and without aura, chronic and nonchronic, with and without medication overuse headache. It would have been useful to match ICHD-3 migraine diagnostic subtype and response rate in addition to the stratification based on the prescribing physician and type of visit. Yet, the mix at US headache centers tends to tilt toward more chronic migraine and more severely disabled patients, which is supported by the baseline characteristics of the patients shown here. The overall positive response rate even in this subgroup of the US migraine patient population is therefore very encouraging.
Conclusions
Real-world clinical data confirm that the effect of REN is consistent across multiple attacks in migraine patients with diverse severity and disability. The data also provide support for the favorable safety profile of REN, suggesting it may offer an alternative effective treatment option with minimal side effects. Therefore, incorporating REN into usual care may reduce medication use and thus decrease the risk of medication overuse headache.
