Acute Treatment of Migraine in Adolescents: Real-World Analysis of Remote Electrical Neuromodulation (REN)
Anna Esparham et al, Pediatric Neurology, 2023
ABSTRACT
Nearly 10% of children and adolescents in the United States experience migraine. Pharmacologic treat- ment of migraine in adolescents is limited due to only few US Food and Drug Administration (FDA)- approved medications, limited efficacy, or lack of tolerability. Remote Electrical Neuromodulation (REN) is a nonpharmacologic abortive treatment for migraine, cleared by the FDA for patients aged 12 years and above. This study evaluated real-world efficacy of REN in adolescents aged 12 to 17 years. Real-world data were collected from patients aged 12 to 17 years treated with the REN device (Nerivio) from January 1, 2021, to May 31, 2022. Study's end points included consistent efficacy two hours after treatment, use of REN as a standalone versus as an adjunct therapy, treatment intensity, and safety. Of 1629 adolescents included in the study, consistent response in at least 50% of treatments at two hours posttreatment was achieved by 60.3% of patients for pain relief, 26.3% for pain freedom, 66.3% for functional disability relief, and 41.2% for functional disability freedom. Of 2365 treatments in which medication usage was reported, REN was used as standalone therapy in 64.4% of the treatments, REN was combined with over-the- counter medications in 18.6%, and it was combined with prescription medications in 17%. Mean treat- ment intensity from 13,716 treatments was 28.5% (±13.6%) of the max stimulator output. Only three device-related adverse events were reported, all minor. This real-world analysis demonstrates the persistent efficacy of REN for abortive treatment of migraine in adolescents, extending findings of prior clinical trials in adolescents and real-world studies in adults.
Introduction
Migraine is a disabling and prevalent disease across the world. In children and adolescents, migraine is a common recurring headache, with up to one of 10 experiencing migraine. As a result, adolescents with migraine have decreased quality of life, increased sleep and behavioral disturbances, more absences from school, and disrupted ability to perform well in school and engage in social activities compared with their peers. Patient well-being depends on the ability to develop an effective treatment strategy for their migraine.
Most standard-of-care acute migraine treatments are pharma- cologic drugs. However, many of them are not approved by the US Food and Drug Administration (FDA) for adolescents. Four types of triptans are approved for abortive treatment of migraine in patients in the 12 to 17 years age segment (rizatriptan, almotriptan, sumatriptan-naproxen, zolmitriptan). However, both over-the- counter (OTC) medications and the migraine-specific triptans have side effects, and if taken excessively, may cause medication overuse headache (MOH) resulting in higher frequency and in- tensity of migraine attacks. As such, noninvasive, better-tolerated efficacious therapies are needed to reduce migraine burden on adolescents.
Remote Electrical Neuromodulation (REN) is a noninvasive, nonpharmacologic alternative to migraine medications for adolescents. The REN device (Nerivio) is authorized by the FDA for acute treatment of migraine in adolescents and adults aged 12 years and above. The device activates an intrinsic pain inhibition mechanism known as conditioned pain modulation (CPM) by stimulating nociceptive nerve fibers in the upper arm in a sub- painful manner.
Previous randomized, double-blind, sham-controlled studies in adults (18 years of age and above) proved REN is safe and effica- cious. A subsequent study found that REN was highly effective, well-tolerated, and safe in the acute treatment of migraine in ad- olescents, and a posthoc analysis comparing REN with standard- care medications demonstrated that REN might be more effica- cious. A large-scale postmarketing study provided real-world evidence (RWE) of the safety, efficacy, and stability of REN for acute treatment of migraine in adults. Data in that study also revealed that REN was used as a single therapy (i.e., standalone treatment) in most attacks.
This study investigates the long-term, real-world efficacy and safety of REN in the acute treatment of migraine in a cohort of adolescents. Study design was largely modeled after the similar RWE study in adults. The study objectives were to evaluate: (1) the consistent efficacy ofusing REN across multiple treatments; (2) the prevalence of using REN standalone versus as an adjunct with migraine medications; (3) the treatment “dosage,” or electrical stimulation intensity applied by the young users; and (4) the safety of device in real-world conditions.
Methods
The Nerivio REN device
The REN device (Nerivio) is a wearable, nonpharmacologic de- vice, controlled by a. smartphone application. Upon the initial manifestation of a migraine attack, patients apply the device to their upper arm for a treatment duration of 45 minutes. The maximum output current of the device is 40 mA, and stimulation intensity is controlled by patients in the device app, with the in- struction to set the intensity to a level that feels strong yet comfortable and not painful.
Study design
The study ( ClinicalTrials.gov NCT05443659) is an RWE anal- ysis investigating the safety and efficacy of REN acute treatment of migraine in adolescents, who were prescribed REN in multiple pediatric neurology clinics across the United States. Data were collected using collection methods similar to those applied in a previous RWE study of REN for abortive migraine treatment in adults, with the eligibility here limited to adolescents (12 to 17 years old). Upon signing up to the Health Insurance Porta- bility and Accountability Act (HIPPA)-compliant Nerivio app, patients accept the terms of use specifying that they provide personal information on their free will, and that research might use their deidentified data. During each treatment, users decide whether to report information regarding their migraine symp- toms and treatment via the in-app migraine diary. At the beginning of every treatment, the app prompts users to report their current pain level (severe/moderate/mild), functional disability (severe/moderate/mild/none limitation), and associ- ated migraine symptoms. Patients are further encouraged to record the same symptoms two hours after the beginning of the treatment and to specify any medications used within that time frame.
Dataset
Data from all adolescent users across the United States who treated their migraine with REN between January 1, 2021, when the FDA approved the Nerivio device for adolescents’ use in the United States, to May 31, 2022, were included in the study. Patient age was determined solely by the date of birth entered upon app registra- tion. Only and all treatments ofat least 30 minutes were included in the analysis.
Outcomes
The following four outcome measures were tested.
(1) Consistent efficacy: To isolate the impact of REN, efficacy was calculated for all evaluable treatments where patients re- ported not taking any medications during the two hours from starting a REN treatment. Efficacy end points included the proportion of adolescents who responded to the following four efficacy metrics consistently, i.e., in at least 50% of their evaluable treatments (therefore, having at least two evaluable treatments): (1) consistent pain relief (decrease from severe or moderate headache level at the time of starting the treatment to mild or no pain at post two hours); (2) consistent pain disappearance (change from severe, moderate, or mild headache at treatment start to no headache pain two hours later);
(2) Usage of abortive (rescue) medications for migraine: The prevalence of REN use in combination with other medica- tions was calculated for each evaluable treatment with re- ported symptoms at both treatment onset and following two hours from treatment onset as well as medications status following two hours. Treatments in the analysis of this end point included evaluable treatments with REN alone (i.e., REN standalone therapy), REN treatments with OTC medi- cation use, REN treatments with oral triptans, REN treat- ments with other prescription medications (the latter three reflecting Nerivio as adjunct therapy), and REN treatments where additional rescue medication use was not reported. The percentage of adolescent users in each category was calculated. Treatments with no report of medication use were not included in the medication analysis breakdown. These definitions are identical to those used in the previous real-world use study of REN.
(3) Treatment intensity (“dosage” ): Stimulation level is manu- ally set via the app by the user during treatment and is automatically recorded during Nerivio treatments. Average mean stimulation level per patient was calculated over all treatments conducted by each patient. In addition, the average intensity distribution is presented as a histogram and compared with the average intensity distribution of treatments in the previous real-world study in adults.
(4) Safety: The entire dataset of REN treatments performed by adolescents was used for the safety analysis. The analysis of all adverse events (AEs) reported during the duration of the study included: total AEs related to the device (DAEs); portion of the DAEs that were severe, moderate, and mild; and portion of serious AEs versus nonserious AEs.
Results
A total of 1629 adolescents included in the study chose to pro- spectively record their symptoms and medication use in the app between January 1, 2021, and May 1, 2021. Symptoms reported in the app were entirely optional, and real-world users who shared their symptoms did so voluntarily. The sample sizes for each end point analysis differed due to variations in the inclusion criteria for each analysis. The average age of study participants was 15.9 years (± 1.3), of whom 80.6% (1313) were female. Age was determined based on date of birth entered when registering to the REN device app. Almost all participants (99.2%, 1616 of 1629) were prescribed in appointments with neurologists and headache providers (and 0.8% by primary care providers, mostly in telehealth appoint- ments). A total of 13,716 REN treatments were performed by the adolescent users. Of the treatments, 97.0% adhered to the full 45 minutes, and the rest were between 30 and 45 minutes duration.
Consistent efficacy over multiple treatments
Data from 582 patients (1524 treatments) in whom REN was used as a single therapy without rescue treatments were used to calculate the four efficacy outcomes. The number of users included in the analysis of this set of end points differed for each end point, based on the number of treatments with reported symptoms. Post two hours consistent pain relief response (i.e., in at least 50% of treatments) was attained by 60.3% (158 of 262) of users, consistent pain freedom by 26.3% (76 of 289), consistent functional disability relief by 66.3% (169 of 255), and consistent freedom of functional disability was attained by 41.2% (105 of 255) of the users (Fig 1). We further conducted a wider analysis, including patients who com- bined REN with acute medications for migraine. This analysis showed similar results, of consistent pain relief reported by 58.3% (233 of 400) of users, consistent pain freedom by 24.1% (105 of 435), consistent functional disability relief by 68.5% (265 of 387), and consistent freedom of functional disability by 36.7% (142 of 387) of the users.
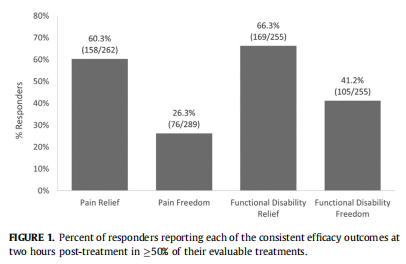
Using REN as a standalone therapy versus as an adjunct
Medication status two hours after treatment initiation was voluntarily reported in 2365 treatments. The number of treatments with reported medication status out of all 13,716 treatments rep- resents a confidence level of 95%, with a margin error of ±1.75%, which is extremely high statistical power. In 64.4% (1524 of 2365) of the treatments REN was the only therapy, in 18.6% (439) REN was combined with OTC medications, in 7.6% (180) REN was combined with oral triptans, and in 9.4% (222) REN was combined with other prescription medications. Efficacy response rates of these sub- groups were similar. This data are presented in Fig 2.
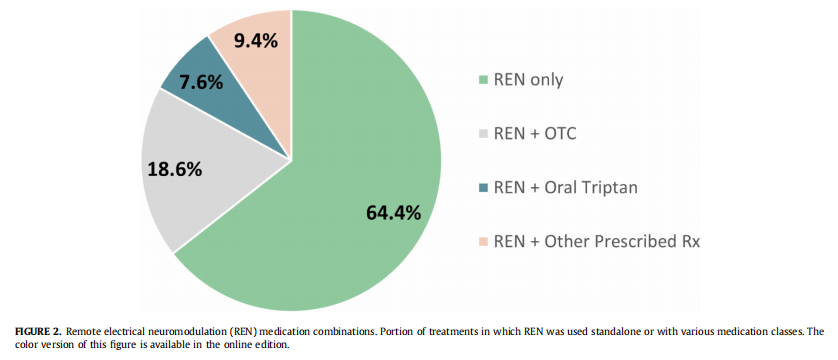
Treatment intensity
The analysis of this end point included all 13,682 treatments conducted by all 1629 adolescents. Average treatment intensity was 28.5% (± 13.6%) of the max stimulator intensity (which is 40 mA), with a median of 26.0%. Intensity ranged between 14% and 46% of the max stimulator intensity for 80% of the users. The average stimulation intensity used in real-world setting by adolescents is slightly lower than what was measured in adults (mean of 28.5% vs 34.3%) and the distribution is shifted leftward relative to that of adults, although the shapes of the two distribution curves are very similar, as can be seen in Fig 3.
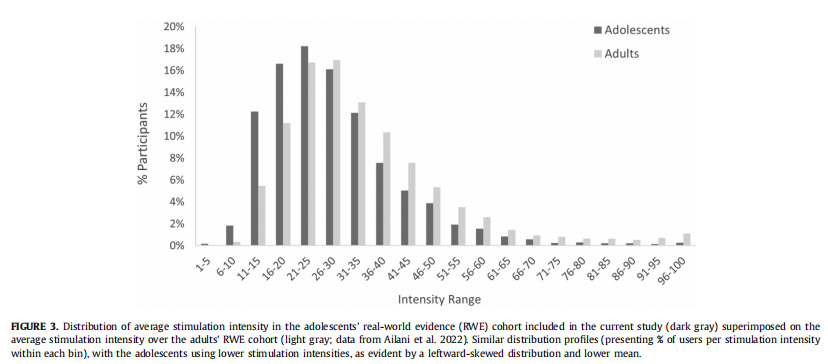
Safety
Of all 13,682 treatments performed by all 1629 participants in the study, three users reported DAE (0.18%). All were minor and not serious. These DAE's included tingling and local soreness during or after treatment. There were no reports of systemic side effects.
DISCUSSION
Previous RWE studies have found that REN is a highly effective and safe nonpharmacologic option for acute treatment of migraine in adults. The current study extends the clinically meaningful benefits to the adolescent population. REN's reliability and usability for acute migraine treatment in adolescents was assessed using four outcome measures.
First, an analysis of efficacy across multiple treatments looked solely on treatments in which REN was the only treatment without the use of any rescue medications (i.e., standalone treatment). Real- world data from adolescents shows that 60.3% of users achieved pain relief from severe or moderate headache to a mild headache or no headache in at least 50% of their evaluable treatments. In addition, 26.3% of responders were pain-free after at least half of their treatments. This analysis also examined improvement in pa- tient's functional ability and found that 66.3% and 41.2% of the adolescents experienced functional disability relief and functional disability freedom, respectively. Although there are some discrep- ancies, with the current data showing slightly higher/lower efficacy than previously reported in both a clinical study of adolescents treating with REN and in real-world data from adults treating with REN, overall, efficacy in the current study is similar to what was previously reported. Similar results were observed in pa- tients who combined REN with another acute medication. The above outcomes imply that the high level of efficacy shown both in well-monitored trials with adolescents and in real-life settings with adults is largely reproduced in real-world use of adolescents as well.
The second measure assessed REN use in combination with standard-care medications; it showed that in nearly 65% of evalu- able treatments, users did not take any medications within two hours of starting REN treatment. In addition, only 17% of users who reported medication status used prescription medications within two hours of REN treatment, suggesting that REN provides enough clinical benefits for adolescents as a standalone treatment option. Previous posthoc analysis from a clinical trial in adolescents showed that standalone REN treatment leads to higher efficacy than standard-care medications in adolescents. Taken together with the current results, combined evidence from both studies proposes that standalone REN can lead to a decrease in the consumption of prescribed medications and can therefore lower the medication overuse headache risk in adolescents. The distri- bution of standalone/adjunct REN treatment in adolescents paral- lels those from the adults’ real-world study in which 66.5% used REN alone and 20.6% used REN together with prescription medi- cations. These results reinforce the openness of both age groups to embrace a nonpharmacologic therapy for acute migraine attacks.
Third, the analysis of treatment intensity in over 13,000 treat- ments from over 1600 adolescent users indicates the general pattern of device use among adolescents. Users are instructed to increase the device intensity to be felt and strong yet nonpainful. Stimulation intensity therefore reflects treatment dosage, equiva- lent to the dosage of a pharmacologic drug. Average treatment in- tensity was 28.5% of the stimulator output, which is lower than the 34.3% in adult real-world use. Similarly, although the shape of the intensity distribution in this study of real-world adolescents is similar to that of real-world data from adults, adolescents use lower stimulator intensities than adults, evident by the left-shift of the distribution (i.e., lower intensities overall). These findings are not surprising given the lower pain threshold at younger ages and the narrower arm circumference and lower body weight of ado- lescents versus adults.
Last, all adverse events were mild, and there were no severe DAEs. The portion of AEs reported by adolescents in real-world setting (0.18%) was lower than in clinical settings (2%). A similar difference is evident in adults, who also report a lower ratio of AEs in real-world setting than in clinical studies. These differ- ences may reflect the request from participants in clinical studies to report every discomfort and event, although in real life, patients tend to reach out only when they are concerned by events.
This study has several limitations. First, this is not a controlled study—it has a single arm, of Nerivio users. Nevertheless, although this implies a difficulty in assessing the results, it is easily compensated using results from numerous clinical trials and RWE studies. Such a comparison demonstrates that results produced in this study are in line with results previously collected with Nerivio.
Second, efficacy and medication data were voluntarily provided by patients through the app, which contributed to the fact that not all adolescent users provided the required information at the beginning of the treatment and following two hours. Nevertheless, complete prospective post-two-hour data resulted in large data- sets—at least 255 participants for each outcome measure—which makes this study considerably large.
Conclusions
The current study expands the findings of previous clinical trials in adolescents and RWE in adults to real-world usage of REN by adolescents, demonstrating that REN is an efficacious, safe, and well-tolerated acute treatment for migraine in adolescents in real- world setting. This study solidifies the importance of REN as a valuable nonpharmacologic acute treatment alternative for ado- lescents either as a standalone therapy or as an adjunct to OTC and/ or prescribed medications. Future research should measure the percentage of patients who refill their prescription and continue using this treatment, albeit this is also influenced by economical/ access considerations.
