Effectiveness comparison of remote electrical neuromodulation and standard-care medications for acute treatment of chronic migraine: a post-hoc analysis
Brian Grosberg et al, pain Management, 2022
Aim: The current study compared the effectiveness of remote electrical neuromodulation (REN) to that of standard-care medications for acute treatments of migraine, using a within-subjects design.
Materials & methods: Post-hoc within-subject analysis was performed on data from 78 adult chronic migraine patients who participated in a clinical trial with REN, on four end points: single-treatment pain relief, single- treatment pain freedom, consistency of pain relief and consistency of pain freedom.
Results: No statistical differences were found between REN and the tested medications, in any of the effectiveness outcomes:single-treatment pain relief p = 0.056, single-treatment pain freedom p = 0.532, consistency of pain relief p = 0.369, consistency of pain freedom p = 1.00.
Conclusion: The results suggest that REN may provide an effective non-pharmacological alternative for standard care abortive medications in individuals impacted by chronic migraine.
Plain language summary: Dueto the high frequency of headaches, patients impacted by chronic migraine are struggling with poor quality of life, as well as elevated risk of medication overuse headache (which might cause migraine chronification). Thus, there is a need for non-pharmacological migraine treatments that are both effective and well tolerated.
Remote electrical neuromodulation (REN) is a non-pharmacological abortive migraine treatment, which is US FDA cleared for adults and adolescents with episodic or chronic migraine. The current study compared the effectiveness of REN to that of standard-care medications (i.e., over-the-counter medications and triptans), using data from 78 individuals with chronic migraine who participated in a clinical trial. During the study, each participant treated their attacks with their preferred medication for the first 4 weeks, and then treated their attacks with REN (only) for the following four weeks. The participants rated their pain level prior to each treatment, and 2 h after the beginning of the treatment. The results indicate no statistical difference between the effectiveness of REN and standard care medications and suggest that REN may provide an effective non-pharmacological alternative for standard care abortive medications, for individuals with chronic migraine.
Keywords: Headache • medication • migraine • Nerivio • neuromodulation • non-pharmacological • pain • relief • REN • treatment
Chronic migraine is a disabling disorder characterized by the occurrence of at least 15 headache days per month, for over 3 months, of which at least 8 days per month have migraine headache features . Due to the high frequency of headaches, patients impacted by chronic migraine are struggling with poor quality of life, frequent absence from work, school and social activities and extensive healthcare utilization intended to manage their pain and associated ymptoms.
The typical treatment plan for chronic migraine consists of both preventive and acute medications. Preventive medications reduce the number of migraine and headache days, but they are unable to prevent all migraine attacks and headache days, requiring the additional use of acute therapies. Additionally, high discontinuation and low adherence rates are reported for preventive treatment due to various reasons. Together, these result in an overuse of acute medications in the chronic migraine population.
Acute medication overuse is a major risk for medication overuse headache, that has become a substantial health concern worldwide. Both over the counter (OTC) analgesics and migraine specific drugs such as triptans can cause medication overuse headache (with prescribed medications imposing a higher risk. Thus, there is a need for alternative non-pharmacological abortive migraine treatments that are both effective and well tolerated. These hold the potential to improve the health and quality of life of patients with chronic migraine.
Remote electrical neuromodulation (REN) is an abortive migraine treatment which stimulates upper arm peripheral nerves to induce conditioned pain modulation – an endogenous analgesic mechanism in which a conditioning stimulus inhibits pain in remote body regions.
Two recent studies demonstrated that REN is an effective and safe abortive treatment for migraine in patients with chronic migraine, adding to previous clinical trials that demonstrated REN’s effectiveness and safety in episodic migraine and in adolescents with migraine. The current report presents a post–hoc analysis of the larger of those recent studies, an open label multicenter clinical trial that examined the effectiveness and safety of REN as an acute treatment for chronic migraine. In the current analysis, the effectiveness of REN is compared, within subjects, to the effectiveness of standard care medications taken during the run-in phase of that trial by the same subjects. The main analysis includes all medications that were taken and subanalyses focusing on OTC and oral triptans are also presented.
Materials & methods
Dataset
The current post–hoc analysis was conducted on data from a prospective, open-label, single arm, multicenter clinical trial, which evaluated the safety and efficacy of REN as an acute treatment of migraine in patients with chronic migraine (NCT04194008) . The clinical trial was approved by Western Institutional Review Board ([WIRB]; approval number 20192678) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants prior to the start of that study.
Reports of migraine/headache symptoms and medication intake were collected via an electronic diary. Pain was rated using a four-level scale of pain intensity: severe, moderate, mild and no pain.
Of the 91 subjects from the clinical trial, 78 (86%) had at least one treatment with medications in the run-in phase and at least one treatment with REN in the intervention phase, for which diary reports were filled before and after the treatment. These 78 subjects comprise the current dataset.
Study design
Medications phase
The first phase of the clinical trial was a four week run-in phase (‘medications phase’). Patients were instructed to use the same medications they usually do, when required and fill the diary before each treatment (i.e., pre-treatment) and 2 h after the treatment (i.e., post-treatment).
REN phase
The next phase of the clinical trial was a four week intervention phase (‘REN phase’). In this phase patients received a REN device (Nerivio, Theranica Bio-electronics Ltd, Israel) and were instructed to treat all their migraines and headaches within 1 h of symptom onset, using the REN device, avoiding any acute medications within 2 h from treatment onset. Treatments in which a migraine medication was taken within the 2 h window between the pre- and post-treatment diary reports were considered a failed treatment (i.e., included in the analysis as no pain relief and no pain freedom).
Outcome measures
Four outcome measures were tested, comparing pre-treatment to 2 h post-treatment:
Single-treatment pain relief defined as a decrease from severe or moderate pain to mild or no pain, or from mild pain to no pain.
Single-treatment pain freedom defined as a decrease from severe/moderate/mild pain to no pain.
Consistency of pain relief defined as pain relief in at least 50% of the subject’s treatments (including all treatments available for that subject, per phase).
Consistency of pain freedom defined as pain freedom in at least 50% of the subject’s treatments (including all treatments available for that subject, per phase).
For the single-treatment analyses, the test-treatment with medications was the first evaluable treatment in the medication phase (evaluable treatment defined as a treatment in which a diary report was completed both before and after treatment). The test treatment with REN was the first evaluable treatment in the REN phase following a training treatment. Given that subjects were using the REN device for the first time, the first treatment in the REN phase was considered a training treatment to allow subjects to get familiar with the device.
In the consistency analyses all evaluable treatments are included.
Analysis
Effectiveness data were compared using the McNemar’s test (suitable for dichotomous within-subject matched reports) and p-values are presented along with an odds ratio ([OR];directionality: OR >1 indicates REN more efficacious than medications, OR <1 medications more efficacious than REN) and a CI. Continuous variables were compared using a paired t-test. Ordinal variables were compared using a Chi-square test. All statistical tests were two-tailed, with statistical significance set at p < 0.05. WinPepi statistics software version 11.65 was used.
In addition to the main analysis that included all medications taken, subanalyses were done, focusing on triptans and OTC medications, separately. For the subanalyses, subjects who reported (separate) treatments with either triptans or OTC, were included in the respective subanalyses datasets (i.e., only treatments in which OTC or triptans were used were included).
Results
Participants
Demographic characteristics of the study sample are presented in Table 1.
Phase characteristics
Characteristics of the study phases, along with their statistical comparison, are presented in Table 2. Rescue medications were taken following REN treatments in 10.5% (51) of the treatments. These treatments were considered as failure of REN, in other words, included in the analysis as no pain relief and no pain freedom, regardless of the patient’s report.
Effectiveness results
Single attack
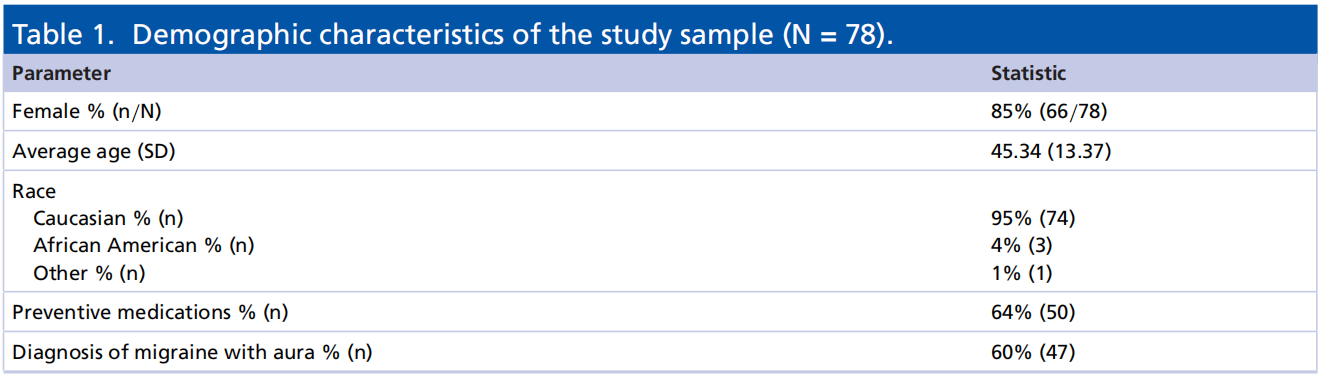
Of the 78 subjects, 62.82% (49/78) reported pain relief 2 h post-treatment when using REN, whereas 48.72% (38/78) reported pain relief when using medications p = 0.056, OR: 2.0 (CI: 0.984–4.063). Post 2 h pain freedom was reported by 23.08% (18/78) of the subjects when using REN, whereas 19.23% (15/78) when using medications p = 0.532, OR: 1.3 (CI: 0.583–2.901); see Figure 1.
Consistency analysis
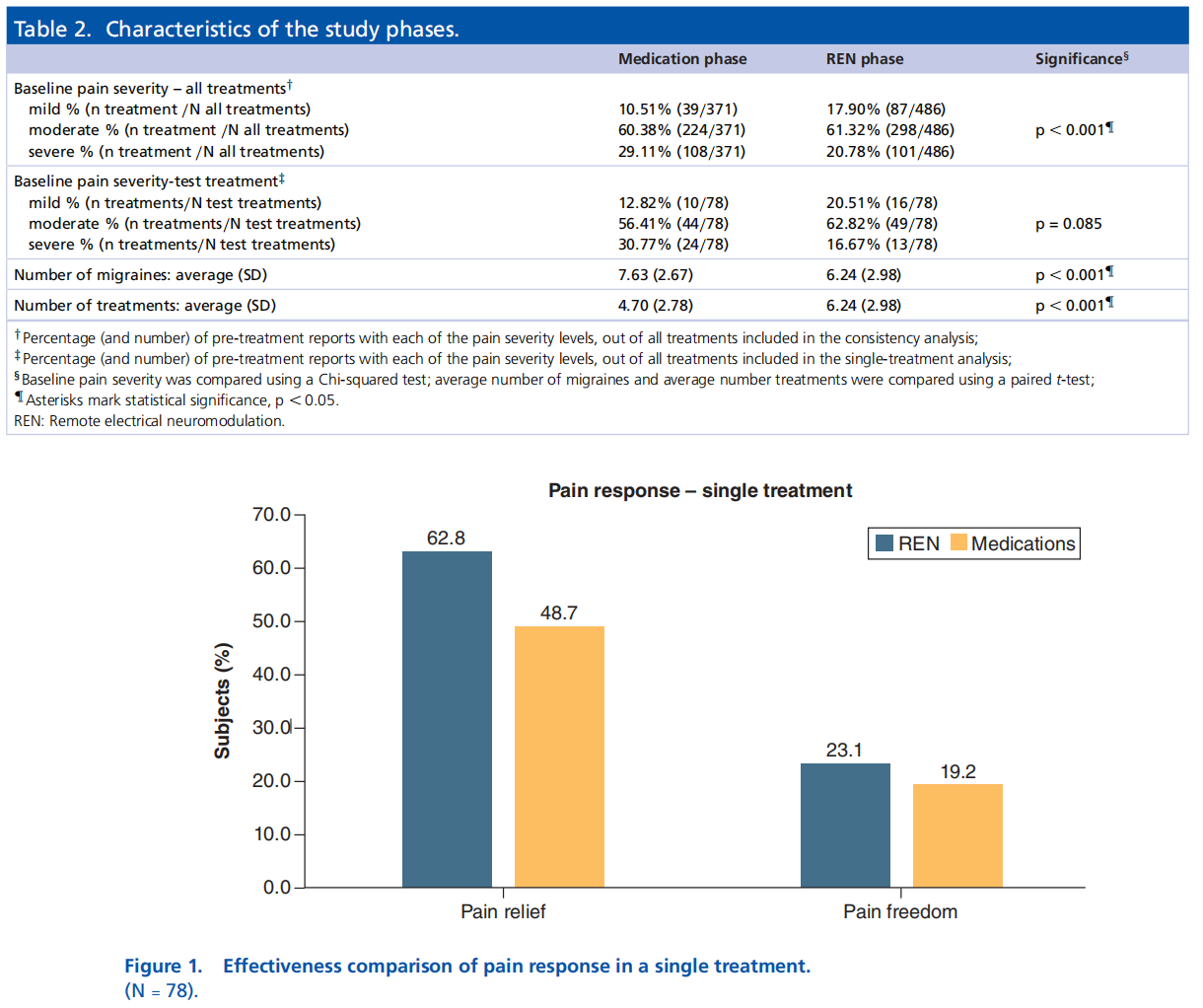
Of the 78 subjects, 64.10% (50/78) achieved consistency of pain relief post 2 h when using REN, whereas 57.69% (45/78) when using medications p = 0.369, OR: 1.4 (CI: 0.688–2.786). Consistency of pain freedom was achieved by the same number of subjects, 14.10% (11/78) when using either REN or medications p = 0.999, OR: 1.0 (CI: 0.389–2.571); see Figure 2.
Sub-analysis by medication class
For an overview of the sub-analyses see Figure 3.
OTC
Single attack
Of the 55 subjects who reported treatments with OTC in the medications phase, 60.00% (33/55) reported pain relief when using REN, whereas 49.09% (27/55) when using OTC medications p = 0.257, OR: 1.5 (CI: 0.736– 3.243). Pain freedom was reported by 21.82% (12/55) of the subjects when using REN, whereas 16.36% (9/55) when using OTC medications p = 0.439, OR: 1.5 (CI: 0.477–5.121).
Consistency analysis
Of the 55 subjects who reported treatments with OTC in the medications phase, 61.82% (34/55) achieved consistency of pain relief when using REN, whereas to 50.91% (28/55) when using OTC medications p = 0.221,
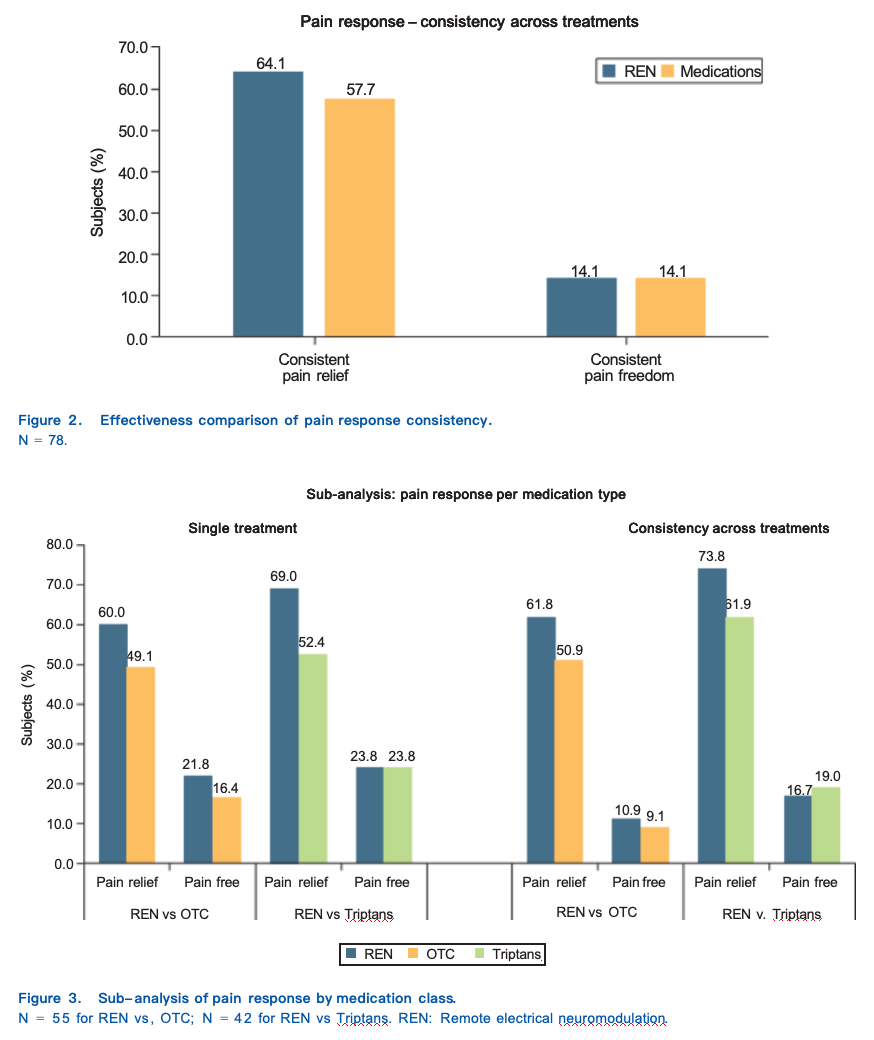
OR: 1.7 (CI: 0.746–3.726). Consistency of pain freedom was achieved by 10.91% (6/55) of the subjects when using REN, whereas 9.09% (5/55) when using OTC medications p = 0.763, OR: 1.2 (CI: 0.389–3.701).
Triptans
Single attack
Of the 42 subjects who reported treatments with triptans in the medications phase, 69.05% (29/42) reported pain relief when using REN, whereas 52.38% (22/42) when using triptans p = 0.071, OR: 2.75 (CI: 0.925–8.176). Pain freedom was reported by 23.81% of the subjects (10/42) when using either REN or triptans p = 0.999, OR:
1.00 (CI: 0.366–2.731).
Consistency analysis
Of the 42 subjects who reported treatment with triptans in the medications phase, 73.81% (31/42) achieved consistency of pain relief when using REN, whereas 61.90% (26/42) when using triptans p = 0.225, OR: 1.8 (CI: 0.704–4.777). Consistency of pain freedom was achieved by 16.67% (7/42) of the subjects when using REN, whereas 19.05% (8/42) when using triptans p = 0.739, OR: 0.8 (CI: 0.233–2.750).
Other medications
Nine subjects used either opioids or other abortive migraine medications in at least one treatment in the medications phase. Given the small size of this group no sub-analysis was performed on these data.
Discussion
There is a significant unmet need for non-pharmacological abortive treatments for patients impacted by chronic migraine. In the current analysis, the effectiveness of REN, a non-pharmacological abortive migraine treatment, was compared with that of standard-care medications (OTC and oral triptans), within subjects. No statistically significant differences were found between REN and the tested medications, suggesting non-inferiority of REN. Notably, in all parameters the OR (ranging 1–2) indicated that the effectiveness of REN was qualitatively at least as good as that of the tested pharmacotherapy.
A subanalysis comparing REN to each of the medication classes, OTC and triptans, separately, further demon- strated results that are similar to the main analysis. The results thus suggest that using REN for acute treatment of migraine in this population may provide a needed alternative, without compromising efficacy.
Importantly, data in the current analysis were collected using a within subject design, with each subject receiving both their own choice of standard-care medication and REN, providing a strong, direct, comparison. The current results resemble previous results from analyses in other migraine subpopulations. A post-hoc analysis that compared REN and standard-care medications for episodic migraine found higher effectiveness of REN compared with medications in single-treatment pain relief, as well as in consistency of pain relief. Similarly, another post-hoc analysis in adolescents with chronic and episodic migraine found higher effectiveness of REN compared with medications in single-treatment pain freedom, as well as in consistency of pain freedom and consistency of pain relief .
The current results resonate with previous reports on the efficacy of OTC and oral triptans. With regards to triptans, the reported efficacies for pain relief at 2 h ranges 42–72% and pain freedom at 2 h ranges 18–50% . The reported efficacy for OTC pain relief at 2 h ranges 37–58% and pain freedom at 2 h ranges 19–25% .
Safety results were presented in detail the clinical trial article from which the current dataset is taken. No serious device related adverse events reported and there was a low rate of device related adverse events (1.0%).
Limitations
First, for each subject, we included all available treatments in each phase. While this was chosen to allow the largest and least filtered possible dataset, it also introduces variability in the number of treatments per person. Second, the average number of treatments per person was higher in the REN phase compared with the medications phase and the pain level before treatments was lower in the REN phase compared with the medication phase. This might be because subjects were part of a clinical trial giving them free access to REN and chose to treat as many attacks as possible with REN in order to test its effect on themselves, as well as more minded to report migraine severity during this phase. However, both these differences pertain only to the consistency analysis and the single-treatment analysis characteristics were not statistically different. Relatedly, the number of reported migraines in the REN phase was lower than in the medications phase. This may suggest a potential preventative effect of using REN, which is now being tested in a dedicated prospective clinical trial (NCT04828707). Finally, the single-attack analysis was performed on the first evaluable treatment in the REN phase following a training treatment. This was done to allow training, given that REN has a unique mode of operation, but could introduce bias toward response (i.e., if non responders chose not to use REN a second time). However, only one subject (out of 79) withdrew from the trial between the first and second treatment, thus the potential impact is limited. Additionally, all evaluable treatments, including the first treatment, were included in the consistency analysis.
Conclusion
The current analysis suggests that REN provides an effective non-pharmacological alternative for standard care abortive medications in individuals impacted by chronic migraine.

