Acute treatment of migraine in children aged 6−11: Real‐world analysis of remote electrical neuromodulation (REN)
Klaus Werner et al, Annals of the Child Neurology Society, 2024
Abstract
Objectives: Migraine is a prevalent neurological disorder severely impacting children and adolescents, yet only one pharmacological treatment is approved for ages 6−12 years. Remote electrical neuromodulation (REN) is a nonpharmacological, prescribed, wearable device cleared by the Food and Drug Administration for acute and/or preventive treatment of migraine with or without aura in patients 12 years and older. This study evaluates REN's safety and efficacy in ages 6−11 years.
Methods: Prospective acute treatment of migraine data were collected through the REN device (Nerivio) smartphone application. Endpoints were device safety (primary); consistent treatment efficacy (headache pain, functional disability, associated migraine symptoms), and REN‐medication combinations 2 h post‐treatment.
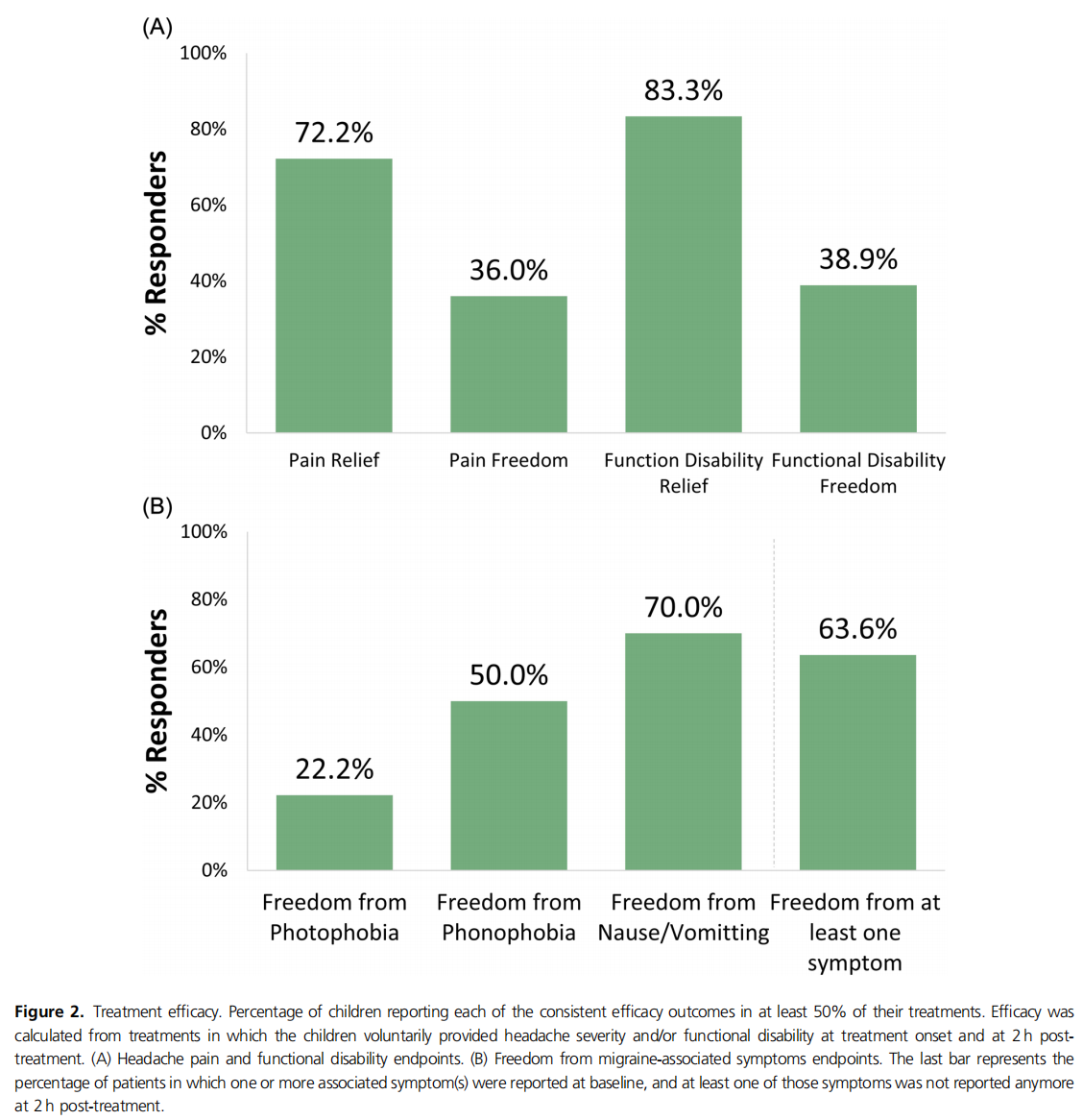
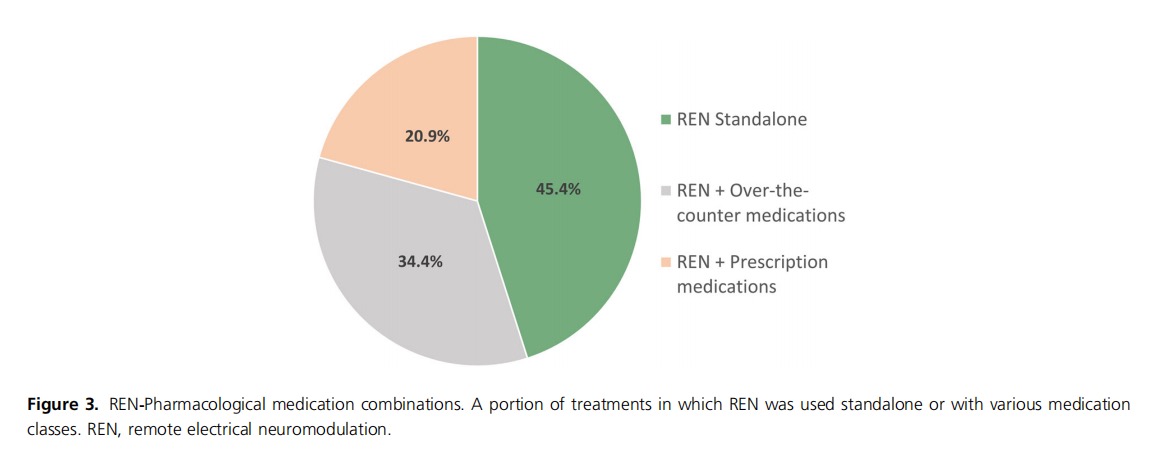
Results: Children (n = 293), median age 11 years (interquartile range = 9−11), 73.7% girls, conducted 5493 REN treatments. No adverse events were reported. Efficacy in at least 50% of REN treatments was calculated from all patients who voluntarily reported pain levels, symptoms, and/or disability at treatment onset and at 2 h post‐treatment, with 72.2% (13/18) of patients reporting pain relief, 36.0% (9/25) pain freedom, 83.3% (15/18) functional disability relief, and 38.9% (7/18) functional disability freedom. Migraine‐associated symptoms disappeared in at least 50% of REN treatments in 70.0% (7/10) of patients for nausea/vomiting, 50.0% (4/8) phonophobia, and 22.2% (2/9) photophobia; 63.6% (7/11) reported freedom from at least one associated symptom. REN was used as a standalone treatment, with over‐the‐counter medications, and with prescribed headache medications in 45.4%, 34.4%, and 20.9% of treatments, respectively.
Interpretation: REN may serve as a safe and efficacious acute treatment of migraine for children. Providers and families seeking a safe, effective, pill‐ and needle‐free treatment option for children suffering from migraine may consider REN.
Keywords: children; headache; migraine; pediatric; remote electrical neuromodulation; REN
Introduction
Migraine, a prevalent and disabling neurological disease characterized by recurrent debilitating headaches and associated symptoms, has a substantial impact on the lives of young children. Affecting up to 10% of children under 12 years of age, its impact transcends the throbbing pain, manifesting in a diverse and age‐dependent phenotype. Younger children, particularly between ages 6 and 12 years, exhibit symptoms like abdominal pain, pallor, anorexia, difficulty thinking, lightheadedness, fatigue, or cranial autonomic symptoms, in addition to the classic symptoms experienced by older individuals, including nausea, vomiting, phonophobia, and photophobia. Migraine attacks significantly disrupt childhood, hindering schooling, social activities, and sleep, leading to a significant reduction in quality of life similar to that of children with rheumatoid arthritis or cancer. In general, headache disorders constitute the second predominant contributor to disability‐adjusted life‐years in adolescents and young adults aged 10−24 years, according to the Global Burden of Disease Study 2019. Moreover, early‐onset migraine, particularly before age 12 years, is a potent predictor of chronic migraine in adulthood, highlighting the crucial need for effective interventions during this critical developmental window. The highest growth in migraine incidence among children, adolescents, and young adults in the last 30 years (1990−2019) occurred in individuals aged 10−14 years.
However, the landscape for the acute treatment of migraine in children aged 6−12 years is extremely limited. Many adult migraine treatments adapted for use in children were not rigorously tested prior to becoming a part of routine care in youth and might not be as effective in children, thus having the potential to present more risk than benefit.16 Rizatriptan is currently the only pharmacological treatment cleared by the Food and Drug Administration (FDA) for ages 6−12 years. However, its efficacy was not superior to placebo in some trials, and its use necessitates careful consideration of dosage and administration due to risk of overuse and potential side effects. While over‐the‐counter pain relievers like ibuprofen, acetaminophen, and naproxen may offer symptomatic relief, they also come with potential side effects for long‐term use. Other pharmacological medications approved for migraine treatment in adults or adolescents are often prescribed off‐label to children, despite limited efficacy and low tolerability. In addition, frequent use of these off‐label treatments may lead to possible medication overuse headaches and chronification of migraine. The lack of efficient treatments for the acute treatment of migraine in children has led to a significant unmet need for efficacious and well‐tolerated acute migraine treatments.
In previous years, noninvasive neuromodulation devices have been added to the arsenal of migraine treatments. Among these, remote electrical neuromodulation (REN) is a nonpharmacological, prescribed, wearable device, FDA cleared for acute and/or preventive treatment of migraine with or without aura in patients 12 years or older. The device triggers an inherent pain relief mechanism called conditioned pain modulation by gently and noninvasively stimulating nociceptive nerve fibers in the upper arm, causing a sub‐painful sensation. Each treatment lasts 45 min, during which the patient can go about their day. The device is attached to the arm with an adjustable armband, which can fit arm circumferences of both boys and girls.
Multiple studies have shown REN's high safety, tolerability, and efficacy in adolescents and adults. Previous double‐blind, sham‐controlled studies in adults have shown REN to be to be safe and efficacious. The safety, effectiveness, and consistent reliability in treating migraine in adolescents aged 12−18 years were further validated in an open‐label clinical trial and in real‐world evidence (RWE) studies. A post hoc study found that adolescents in the acute migraine clinical trial of REN reported higher rates of benefit with REN compared with their standard‐ care medications.
This study aims to evaluate REN's real‐world safety and efficacy in the acute treatment of migraine in children aged 6–11 years.
METHODS
The REN device
The REN device (Nerivio; Theranica Bio‐Electronics Ltd.) is an FDA‐cleared prescribed, self‐administered device indicated for acute and/or preventive treatment of migraine in patients 12 years of age or older. It is a wearable, smartphone‐controlled, nonpharmacological, noninvasive device applied to the upper arm. The device is thin and lightweight, secured to the upper arm with an adjustable armband. Given their small arm circumference, children usually use the extra small (XS) or small (S) armbands (Figure 1).
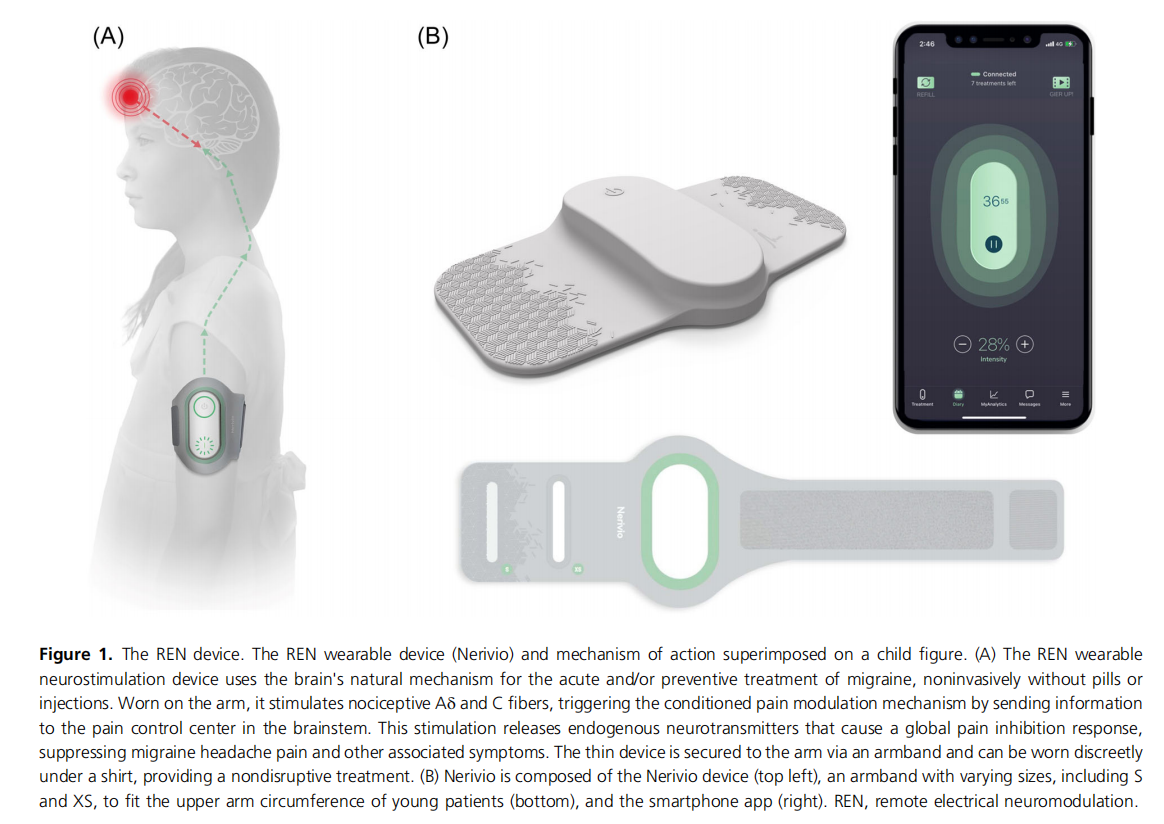
The device uses a preparatory modulated, symmetrical, biphasic, square pulse with a pulse width of 400 ms, modulated frequency of 100−120 Hz, and up to 40 mA output current. At the beginning of treatment, patients are instructed to set the treatment intensity representing the output current, via the smartphone app, to a subjective level that is strong but not painful by increasing or decreasing the intensity with ± buttons in the app. It takes a minute to put on the device and set the treatment intensity, followed by 45 min of treatment, during which patients may continue with their normal daily activities (studying, playing sports, participating in social activities, sleeping, etc.); they are only refrained from immersing the device in water (e.g., shower, pool). The slim profile of the wearable device, which can be covered by a shirt, and the smartphone control make it easy to use it discreetly. Similar to a pack of pills with a set number of pills, each device unit has 18 treatments, as determined by the battery. Patients can order a refill device by pressing the refill button in the app.
Participants and data collection
This RWE study analyzes prospective data from children aged 6−11 years in the United States who treated their migraine attacks with the REN (Nerivio) device at least once between May 2020 and December 2023.
Given that the indication for Nerivio is patients aged 12 years and above, all study participants were prescribed the device off‐label by neurologists and pediatric headache specialists in US children's hospitals and pediatric neurology clinics.
Upon signing up for the Health Insurance Portability and Accountability Act (HIPAA)‐compliant Nerivio app, patients (and, because all of these patients are younger than 18, their parents), accept the terms of use specifying that personal information is provided on their free will, and that research might use their deidentified data. Data were collected prospectively through the REN device (Nerivio) smartphone application, similarly to what was done in previous RWE studies of REN. REN treatments are automatically registered in secured and deidentified accounts in the Nerivio database. At treatment onset and at 2 h post‐treatment, patients are prompted with a series of questions, asking them to voluntarily report their prospective migraine symptoms (headache pain severity, associated symptoms) and whether they used rescue medications. Patients may skip or disregard the questions, which are not required to use the REN device. Both questionnaires need to be completed for efficacy calculations. When answered, the information is prospective. Treatment intensity is automatically recorded throughout treatments and therefore captured from all patients. These data are then presented in a graphical summary and on a monthly calendar in the app to allow patients (and their parents, in the case of children aged 6−11 years old) to track their migraine and treatment patterns. All data are saved to the HIPAA‐secured REN server.
Data analysis and endpoints
The first treatment of each participant was considered a training treatment and was only included in the safety analyses.
Primary endpoint: safety. Device safety was measured using an adverse event tracking system. An additional analysis assessed the average number of device‐related customer support inquiries per patient (including adverse events, device operational issues, educational questions, and effectiveness). The average number of inquiries per patient was compared between the current cohort of children younger than 12 years of age versus all other REN patients.
The following secondary endpoints were calculated:
1. Efficacy (change in headache pain, functional disability, associated migraine symptoms). Efficacy endpoints were calculated from all users who voluntarily provided prospective reports of migraine symptoms at treatment onset and at 2 h post‐treatment reports. Consistent efficacy was conducted in cases where at least two treatments were performed. Patients who experienced the outcome (e.g., pain freedom) in at least 50% of their reported treatments, during which they did not report the use of rescue medications, were considered responders. Headache pain and functional disability were rated at baseline and at 2 h post‐treatment on a 4‐ point scale: severe, moderate, mild, or none. Migraine‐ associated symptoms (photophobia, phonophobia, and nausea/vomiting) were marked as present or not. Pain relief was calculated as a decrease from severe or moderate levels at baseline to mild or none at 2 h post‐ treatment. Functional disability relief was calculated as any improvement in disability from baseline to 2 h post‐ treatment. Pain/functional disability freedom was calculated as a decrease from any pain/disability level at baseline to none at 2 h post‐treatment. Freedom from a specific migraine‐associated symptom was calculated as the percentage of patients reporting the presence of that symptom at baseline and the lack of that symptom at 2 h post‐treatment. Freedom from at least one associated symptom was calculated as the percentage of patients in which one or more associated symptom(s) were reported at baseline and the lack of at least one of those symptoms at 2 h post‐treatment. The number of patients varies between analyses, given the different predefined criteria of baseline and 2 h post‐treatment reports.
2. REN‐medication combinations. REN‐medication combinations were calculated from all users who voluntarily provided 2 h post‐average treatment medication reports. Treatment patterns of REN use as a standalone treatment versus in combination with medications were extracted from these reports as the percentage of treatments in which no rescue headache medications were used (REN standalone) or in which REN was used with over‐the‐counter medications or with prescription medications.
3. Treatment intensity. Treatment intensity (stimulation current) is set by patients via the smartphone app and automatically recorded. The average intensity over all treatments per patient was calculated to provide personal intensity levels, which was used for the following cohort analyses. Average personal intensity levels and the range for 80% of the users were calculated over the patient cohort. Intensity distribution across patients is presented as a histogram and compared with the average personal intensity distribution of adolescents (aged 12−17 years) and adults (age ≥18 years) measured in previous real‐world studies.
Mean and standard deviation were used for variables with a normal distribution, and median and interquartile range (IQR) were used for variables not meeting normal distribution.
Results
Participants
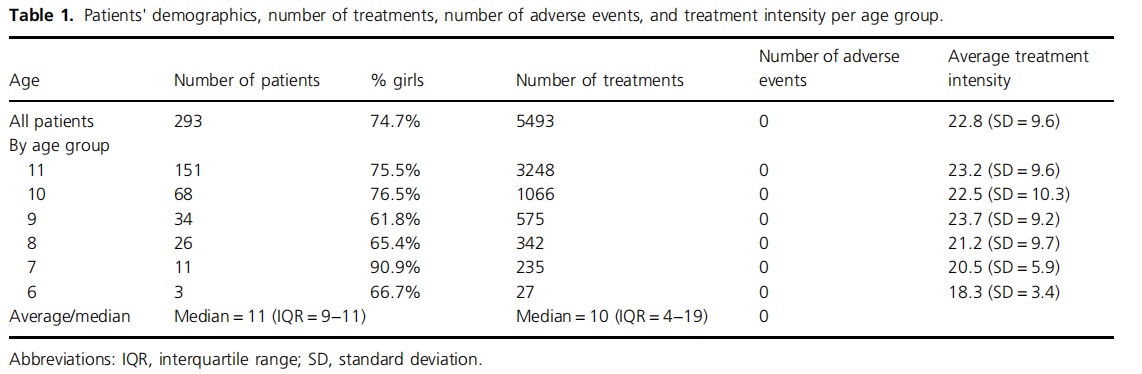
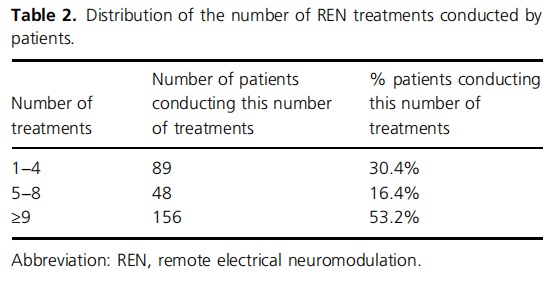
Two hundred and ninety‐three (n = 293) children (73.7% girls) used the REN device to treat at least one migraine attack and were therefore included in the study. Of them, 18.4% reported at least one attack with aura, and the remaining 81.6% never reported aura. Patients' ages ranged between 6 and 11 years of age, inclusive, with gradually more patients in the older age groups (median = 11, IQR = 9−11; see Table 1). Patients performed a total of 5493 REN treatments, ranging between 1 and 499 treatments per patient. Less than one‐third of the patients discontinued the therapy after a few treatments (30.4% conducted one to four treatments), 16.4% continued and conducted five to eight treatments, and most patients (53.2%) conducted nine or more treatments (Table 2). Two‐thirds (67.4%) of REN treatments were not followed by another REN treatment within 24 h, indicating no retreatment and implying no recurrence within 24 h. Note that since Nerivio is also used for prevention (used every other day) by some of the patients, of the 32.6% of treatments that were followed by another treatment within 24 h, one of the two treatments (either the first or the second) could have been conducted for migraine prevention while the other was for attack abortion.
Safety
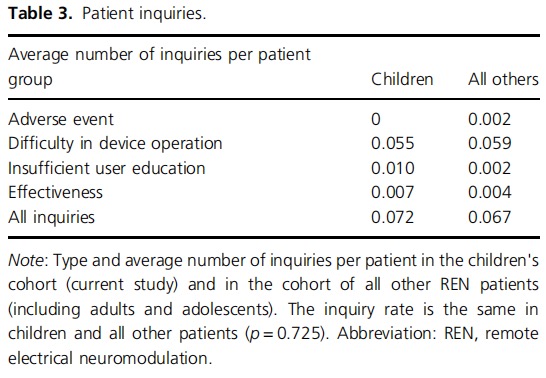
No adverse events and no device‐related adverse events were reported. An additional analysis assessed the number of device‐related customer support inquiries conducted by this patient cohort (usually through their parents), showing an average of 0.072 inquiries per child, which is similar to the inquiry rate of the entire population of REN users of all ages (0.067, p = 0.725; Table 3).
Efficacy
Data from all patients who voluntarily reported treatment results at 2 h post‐treatment were used for this analysis. Consistent efficacy in at least 50% of their treatments (Figure 2A) was reported by 72.2% (13/18) of the patients for pain relief, 36.0% (9/25) for pain freedom, 83.3% (15/18) for functional disability relief, and 38.9% (7/18) for functional disability freedom. Consistent 2 h post‐ treatment freedom from associated migraine symptoms in at least 50% of the treatments per patient in which the patient reported an associated symptom at baseline (Figure 2B) was 70.0% (7/10) for nausea/vomiting, 50.0% (4/8) for phonophobia, and 22.2% (2/9) for photophobia. Freedom from at least one associated symptom was reported by 63.6% (7/11) of patients.
Combining REN with medications
The use or avoidance of a rescue medication to treat headache pain was voluntarily reported in 304 treatments. Of these, REN was used as a standalone therapy in 45.4% (137/304) of treatments, and patients avoided using a prescribed migraine medication in 79.8% of the treatments (241/304). REN and over‐the‐counter medications were combined in 34.4% (104/304) of the treatments, and REN was combined with another prescribed medication in 20.9% (63/304) of treatments with 2 h post‐treatment reports (Figure 3).
Treatment intensity
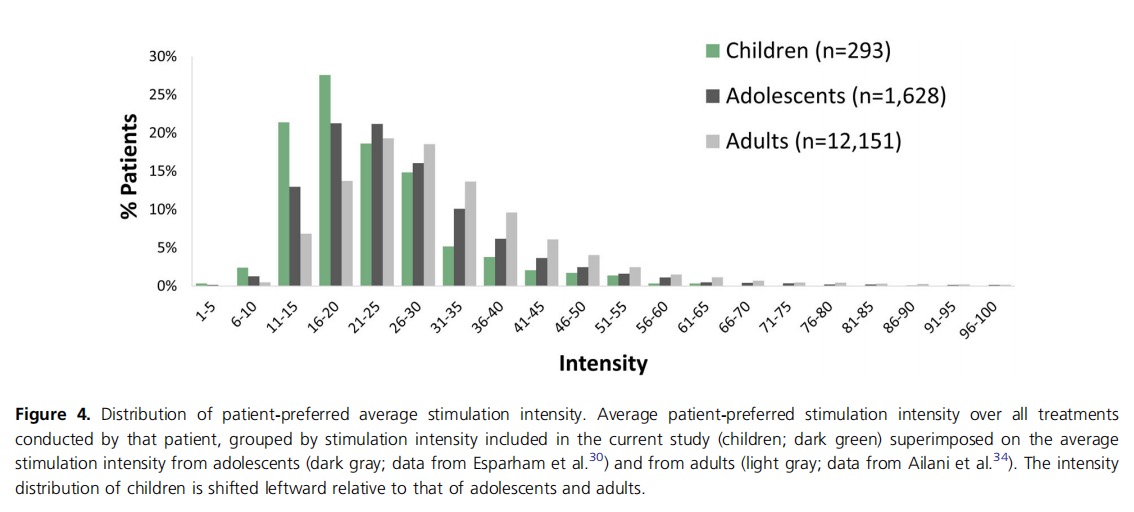
The average treatment intensity across patients was 22.8% (±9.6%) of the max stimulator intensity (40 mA), with a median of 20.5%. Intensity ranged between 13.0% and 35.3% of the max stimulator intensity for 80% of the treatments (between the 10th and 90th percentiles).
The statistics of preferred personal stimulation intensity (over all treatments conducted by a patient) used in a real‐ world setting by children is similar yet slightly lower than what was measured in 1628 adolescents (mean of 26.7 ± 12.2), which was lower than the average intensity used by 12 151 adults (31.0 ± 13.5), respectively.30,34 The intensity distribution among children is shifted downward (left) relative to that of adolescents, with a clear peak in the 16%−20% intensity bin, although the shapes of the two distribution curves are similar (Figure 4).
Discussion
This RWE study in 293 children showed that REN is a safe nonpharmacologic migraine treatment in ages 6−11 years. These results add to the large body of evidence showing the effectiveness and safety of REN in controlled clinical trials in adults, in a clinical trial in adolescents (aged 12−17), and in RWE studies in these age groups (adults and adolescents). Our study in children used similar real‐world methodology and outcome measures as in the real‐world studies in older patients.
The high safety profile of REN in children is evident, given that no adverse events and no device‐related adverse events were reported by children using REN. The lack of adverse events does not reflect an avoidance of patients in this age group (and/or their caregivers) to reach out with other inquiries or complaints related to the treatment, as an additional analysis showed no difference in the number of customer inquiries by children (or their caregivers) and all other (older) REN users, in other age groups.
The efficacy analyses focused exclusively on treatments involving REN as the singular intervention without the concurrent use of any rescue medications, essentially constituting a standalone treatment. When using REN alone, without any rescue medication, nearly 3 of 4 (72.2%) children achieved relief from severe or moderate headaches, reducing them to mild or no headaches in at least 50% of their treatments. Furthermore, more than 1 of 3 children (36.0%) experienced complete post–2 h pain freedom in at least 50% of their treatment sessions. Importantly, REN is associated not only with the improvement or disappearance of headache pain but also with the disappearance of associated migraine symptoms. At 2 h, 70.0% of patients experienced a cessation of nausea/ vomiting. Photophobia and phonophobia disappeared in half (50.0%) and in nearly a quarter (22.2%) of the children, respectively. Overall, more than half of the children (54.5%) experienced the disappearance of at least one associated symptom that was present at baseline—nausea/vomiting, photophobia, or phonophobia—at 2 h post‐treatment.
These efficacy rates of REN are similar to or higher than those in a randomized trial of rizatriptan in children and adolescents and in the 6−11 age group in a larger randomized trial, with 74% and 54% 2 h pain relief, and 31% and 40% 2 h pain freedom, respectively. Yet the efficacy from REN reported in this study comes without the systemic adverse events that were reported in these trials, including asthenia/fatigue, dizziness, somnolence, dry mouth, and nausea. Other randomized trials of adolescents using sumatriptan, zolmitriptan, and oraleletriptan demonstrated safety but not efficacy for triptans in this age group.
Assessing functional improvements, more than 4 of 5 children (83.3%) experienced relief from functional disability, and more than 1 of 3 children (38.9%) returned to full functional ability despite the migraine attack they experienced 2 h beforehand. Given the significant disruptive impact that migraine has on the quality of life of children, perceived to be similar to that of rheumatoid arthritis or cancer at this age,8 having a safe treatment that leads to an improvement or even full return to normal function 2 h from treatment onset is critical for their childhood.
Results from the current cohort of children show similar rates of efficacy in achieving pain relief, pain freedom, and functional relief at 2 h post‐treatment in at least 50% of their treatment sessions compared with RWE in adolescents (72.2%, 83.3%, 36.0% in children vs. 60.3%, 66.3%, 26.3% in adolescents, respectively).
As with many migraine treatments, REN can be used either as a standalone treatment or as part of a combination therapy, for example, with standard‐care medications. Nearly half (45.4%) of the children undergoing REN treatment did not take any medications and used REN as a standalone treatment. REN was combined with over‐the‐ counter medications in a third (34.4%) of the treatments. Thus, patients chose to avoid prescribed migraine medications in 79.1% of these treatments—that is, in only a fifth (20.9%) of treatments, REN was combined with a prescribed medication. This finding is similar to the low percentage (17.0%) of using REN with other prescribed treatments in adolescents. The current clinical evidence that REN alone can provide significant therapeutic benefits without any adverse events provides clinicians with a considerable treatment option, especially given the risk of adverse events reported by children using the abeled or off‐label pharmacological medications commonly used today.
REN users are instructed to set the treatment intensity to be strong yet comfortable and not painful. Thus, each patient finds their optimal intensity by using the app controls. The patient‐preferred average intensity is calculated over all treatments conducted by each patient. The average of treatment intensities as used in real‐life treatments by children was 22.8% (±9.6%) of the maximum stimulator intensity (which is 40 mA), with 80% (between the 10th and 90th percentiles) of the preferred personal intensities falling between 13.0% and 35.3% of the maximum stimulator intensity. Notably, treatment intensities used by children peak in the lower 20s and are a little lower than those used by adolescents,30 showing a leftward distribution shift relative to that of adolescents, although both distribution curves have an overall similar shape with a long tail in the high‐intensity range (Figure 4). This finding is well expected, given (a) the lower pain threshold at younger ages40 and (b) the smaller arm circumferences in children than in adolescents and adults. Throughout the clinical trials of REN, a statistical correlation was observed between larger arm circumferences and higher stimulation intensities preferred by patients. Given the current results, the same instruction should be given to children, namely to “set the treatment intensity to be strong yet comfortable and not painful,” with a recommendation to try and use intensities above 16% of the device stimulator, as was successfully used by most (75.9%) children in this cohort.
The number of REN treatments varied between children. Most patients (53.2%) performed nine or more REN treatments, which is more than the number of pills in most triptan packages (nine pills per pack, typically), while 30.4% performed only four treatments or fewer. One cause for the different number of treatments performed by patients emerges from the fact that, as an RWE study, patients have been prescribed REN at varying time points prior to the conduction of the analysis. Another possible cause for the variability in the number of treatments may reflect different levels of satisfaction. While no adverse events were reported, patients could have discontinued the treatments due to discomfort (which may not be reported as an adverse event) or due to lack of efficacy. The 30.4% of children who performed one to four treatments may reflect discontinuation by those who do not experience clinical benefit or are intolerable to the electrical stimulation of the REN device, similar to the general population of REN users.
There are several limitations to this study. First, it is not a controlled trial, and it involves a cohort of patients using the Nerivio device. Therefore, it might be more difficult to interpret the placebo contribution. Nevertheless, the observed findings align with findings in previous REN controlled clinical trials as well as in other REN RWE studies.
Second, while efficacy rates are high, not all children provided data for efficacy calculations. REN efficacy is calculated from voluntarily in‐app reports of symptoms and use/avoidance of rescue medications at both treatment onset and at 2 h post‐treatment. Therefore, because baseline and post‐treatment reports were voluntary, the fact that the data were collected from real‐world treatments (as opposed to a structured clinical trial), and that many children in this young age of 6−11 years do not have their own smartphones and use their parents' smartphones to administer the REN treatment, it is not surprising that the children go about their day once they achieve pain freedom or relief from their migraine attacks and don't bother to provide reports. Moreover, the fact that most patients (76.1%) continuedtreating four or more treatments even without providing symptom reports by itself represents satisfaction. This further highlights the importance of guiding patients to use the REN device—the same as with other acute treatments of migraine—a few times before their parents or caregivers decide whether their child should continue using it.
Third, this cohort includes all children under the age of 12 years who were treated with REN until the time of data analysis. The age among the cohort of children is not uniformly distributed, with more children in the older subgroup than the very younger subgroup of 6−7 years of age. This is not surprising, given that the incidence of migraine increases with age. More data are continuously collected from all children treating with REN, and therefore larger cohorts of young children are expected to be available for future studies.
A future study assessing REN for migraine prevention in this age group is recommended. The Childhood and Adolescent Migraine Prevention study found no significant difference between topiramate, amitriptyline, and placebo in reducing migraine headaches in children. These two medications had greater side effects than the placebo, and serious adverse events occurred in each medication group. In a more recent systematic review and network meta‐analysis of double‐blind randomized controlled trials with pediatric migraine patients, none of the investigated drugs demonstrated convincing evidence that they reduce migraine frequency in the long run more than a placebo. Propranolol and topiramate, both off‐label for children under the age of 12 years, showed mixed results compared with placebo. Together, these findings suggest that prophylactic pharmacologic treatments have little evidence supporting efficacy for pediatric migraine.
While there are new treatments for migraine prevention in older patients, their safety (or efficacy) for this age group has not been evaluated. Some migraine prevention medications, such as onabotulinumtoxinA and monoclonal antibody injections, further burden children with the delivery via injections. The fear of needles is challenging and common among children and adolescents, with up to 63% of children reporting a fear of needles. REN is already approved and in clinical use for the acute and/or preventive treatment of migraine in patients aged 12 years or older. The usage for prevention is every other day, and the primary efficacy endpoint used in its clinical trials is the reduction in mean migraine days per month. While not measured directly in this study, data available from other studies of REN31 support the safety and tolerability of REN for migraine prevention in children, with a frequent treatment pattern observed in some of the patients in our cohort.
Conclusions
Current guidelines from the American Academy of Neurology and American Headache Society prioritize nonpharmacological interventions in the pediatric age group, including lifestyle modifications, stress management techniques, and cognitive behavioral therapy. While effective in reducing attack severity and frequency, these strategies may not be sufficient for all children, especially not for acute treatment.
Our results align with previous clinical trials and RWE in adolescents and adults, confirming the efficacy, tolerability, and safety of REN in the treatment of acute migraine, demonstrating no adverse events and high efficacy in children. Providers seeking a safe, effective, pill‐free and needle‐free treatment option for children 6−11 years of age who are suffering from migraine may consider REN.
