Coverage With Evidence Development Study Shows Benefits in Patients With Migraine Treated With Remote Electrical Neuromodulation
Andrea Synowiec et al, THE AMERICAN JOURNAL OF MANAGED CARE, 2025
Migraine is a disabling headache disorder affecting 15% of adults and approximately 5 million children and adolescents in the US. Migraine imposes a significant financial burden on individuals and the health care system. A 2016 study estimated the total annual cost associated with migraine to be $36 billion, with direct health care costs accounting for approximately 74%. Millions of individuals in the US seek safe, effective, and accessible migraine therapies. Migraine treatments fall into 2 categories: acute treatments to abort migraine attacks and preventive treatments to reduce their frequency. Traditional medications for migraine include over-the-counter (OTC) and prescribed analgesics, oral triptans, β-blockers, antidepressants, and anticonvulsants. New medication classes, such as calcitonin gene-related peptide (CGRP) antagonists, nasal sprays, and injections, have been introduced in recent years. Yet pharmacological treatments frequently yield incomplete responses, leading to migraine chronification and increased health care utilization. Although newer migraine medications such as CGRP monoclonal antibodies and small molecule antagonists (gepants) offer migraine relief with potentially fewer adverse effects, long-term effects such as hair loss are still being evaluated, and they may not be affordable or approved for all patients.
The remote electrical neuromodulation (REN) wearable (Nerivio; Theranica) is an FDA-cleared, drug-free, noninvasive device for treating both acute and preventive migraine in patients 8 years and older. It delivers electrical stimulation to the upper arm, triggering conditioned pain modulation (CPM). The REN wearable offers migraine treatment, especially for individuals for whom traditional medications are unsuitable due to contraindications, drug-drug interactions, poor tolerability, or lack of efficacy, as well as special populations of children, adolescents, and pregnant women. Extensive research has demonstrated the safety and efficacy of the REN wearable for migraine treatment, including results from multiple randomized controlled trials (RCTs), open-label trials, survey studies, drug comparison studies, and real-world evidence studies. Independent systematic reviews and metaanalyses25,26 have also evaluated the device, and it is endorsed by the American and French headache societies.
This coverage with evidence development (CED) study evaluated the medical necessity and health economics advantages of covering the REN wearable for members of Highmark Inc, a major US health insurer. The current article examines the effectiveness, safety, and utilization of REN for members with migraine who did not respond to previous medications and were prescribed the REN wearable as part of their routine clinical care. The primary hypothesis was that REN treatment would result in significant improvement in migraine related disability. Therefore, reduction in the Migraine Disability Assessment (MIDAS) score was prespecified as the primary end point.
ABSTRACT
OBJECTIVE: Migraine affects millions of individuals in the US, resulting in high health care costs and productivity loss. Traditional medications are often limited in effectiveness and tolerability, creating a need for accessible nonpharmacologic options. This coverage with evidence development (CED) study assessed the necessity of the remote electrical neuromodulation (REN) wearable device for migraine treatment as a standard payer-covered treatment.
STUDY DESIGN: Real-world postmarketing CED study in 2 clinics for 14 months.
METHODS: Members (aged 12-75 years) of a major US health insurer (Highmark Inc) diagnosed with migraine were prescribed REN as part of their clinical care. Effectiveness was evaluated by change in Migraine Disability Assessment (MIDAS) score from baseline to 3 months of treatment and by prospective pain and disability reports 2 hours post treatment. Utilization was measured through prescription fulfilment and safety via adverse event reports.
RESULTS: A total of 381 patients (mean [SD] age, 40.5[13.2] years; 91.1% female) participated. Change in MIDAS score was calculated from all participants who answered the questionnaire twice (n=116), showing a significant and clinically meaningful mean (SD) improvement of –12.1 (51.8) points (P=.014), from 58.3 (59.0) to 46.2 (44.1). Of the participants, 77.8% reported pain relief and 33.3% reported pain freedom; 70.6% and 50.0% reported relief and freedom from functional disability, respectively. Patients used a mean (SD) of 4.0 (3.1) devices annually (extrapolated). Three minor adverse events were reported. These positive outcomes led to the inclusion of REN as a standard treatment for migraine under Highmark policy.
CONCLUSIONS: REN leads to significant clinical and functional benefits in patients with migraine. Additional health insurers are encouraged to consider REN as a standard covered treatment.
TAKEAWAY POINTS
This research highlights the effectiveness and real-world applicability of the remote electrical neuromodulation (REN) wearable device, an FDA-cleared neuromodulation therapy, in reducing migraine-related disability. Findings inform managed care decision makers about REN’s value as an accessible, drug-free treatment option, supporting its integration into clinical practice guidelines and health care policies for migraine management.
› REN is an FDA-cleared, noninvasive, drug-free neuromodulation device for acute and/or preventive migraine treatment in patients 8 years and older.
› Treatment with REN significantly improved participants’ Migraine Disability Assessment (MIDAS) scores, demonstrating clinical and functional benefits.
› Study results led to a positive coverage decision by the payer whose members participated, establishing REN as a covered standard-of-care treatment for patients with migraine.
METHODS
Study Design
This real-world postmarketing study presents a CED program sponsored by a US health plan, Highmark. The study included patients from 2 clinics in Pennsylvania: Allegheny Health Network in Pittsburgh and Penn State Health in Hershey. During the study period, November 2022 to February 2024, patients insured by Highmark who were found to be eligible for the CED program (see criteria below) received REN coverage for 12 months at no out-of-pocket cost.
Participants
Highmark members aged 12 to 75 years diagnosed with migraine were eligible for the study if they met at least 1 of the following criteria: (1) at least 1 standard-of-care acute migraine therapy had failed for 1 or more of the following reasons: contraindication, lack of sufficient efficacy, or intolerability of adverse effects; (2) were at risk of drug-drug interaction with other medications; (3) were a pregnant woman, woman trying to conceive, or breastfeeding woman; (4) had chronic migraine and were at risk of or diagnosed with medication overuse headache (MOH); or (5) were younger than 18 years. Patients with uncontrolled epilepsy, active implantable electrical devices, congestive heart failure, severe cardiac disease, or cerebrovascular disease were excluded. All participants treated with the REN wearable device at least once.
The REN Device
The REN wearable (Figure 1) is an FDA-cleared, noninvasive, drugfree, prescribed neuromodulation device for acute and/or preventive treatment of migraine in patients 8 years and older.30 The device, described in depth in previous studies, is controlled by a smartphone application (Nerivio). A proprietary electrical signal activates small nociceptive nerves in the skin, activating the CPM mechanism and initiating a pain inhibition process. Patients are instructed to adjust the intensity of the treatment so that the stimulation is well felt yet not painful. Each device lasts for 18 treatments of 45 minutes each, during which the patient can go about their day. Once all 18 treatments are used, the patient disposes of the device. A new device is ordered and shipped for continuous treatment.
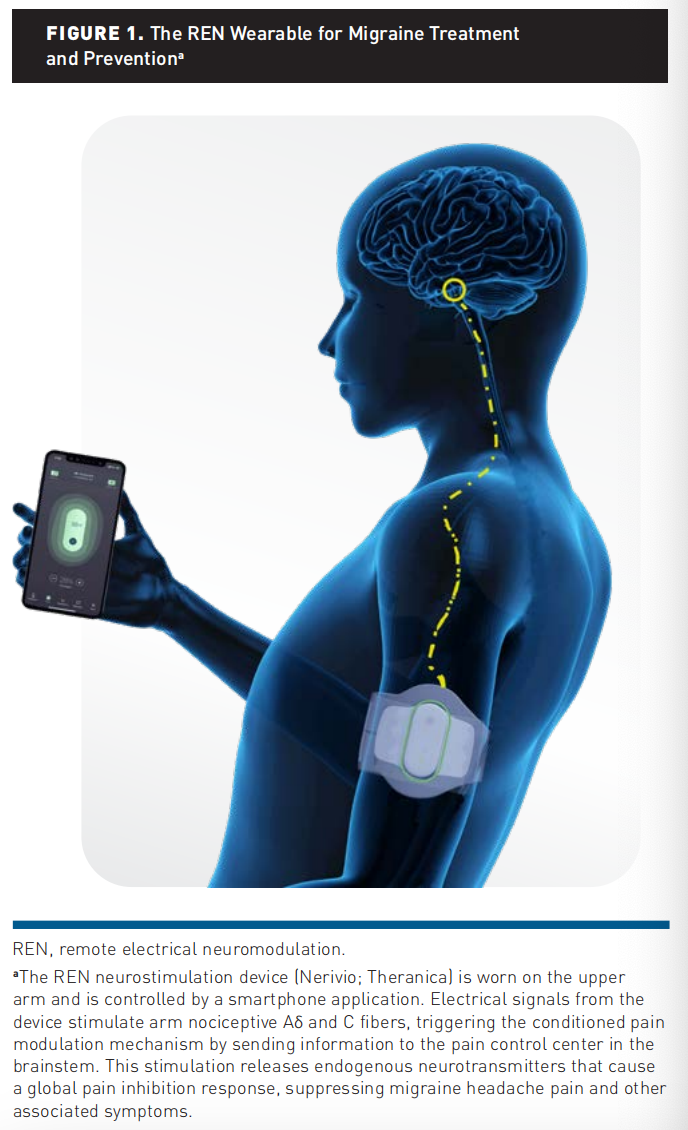
Ethics
As part of the sign-up process to the Nerivio application, all patients accepted the terms of use, acknowledged that providing personal and clinical information was voluntary, and consented for their deidentified data to be used for research purposes. As a postmarketing CED study, this did not require institutional review board approval.
Procedures
Participating health care providers prescribed eligible patients the REN wearable for acute and/or preventive migraine treatment. Patients were asked to complete the MIDAS questionnaire in the clinic to assess the impact of migraine on their life during the previous 3 months (ie, prior to using REN) as a baseline measure of quality of life. Three months post treatment initiation (and in some cases up to 5 months), 2 questionnaires were administered online or over the phone: the MIDAS questionnaire and a treatment satisfaction and tolerability questionnaire (TSTQ). Study personnel made up to 3 attempts to collect post–3-month data. Additionally, at the beginning of each treatment and 2 hours post treatment, patients were prompted by the Nerivio application to voluntarily record their pain level, functional disability, and medication intake.
Sample Size
The study aimed to enroll a mix of patients with high-frequency episodic migraine and chronic migraine, based on previous research showing mean MIDAS scores of 20 and 35 points, respectively. Based on the expected migraine severity (mean [SD] MIDAS score, 30[10]), MIDAS scores in a previous REN study, and the minimal important change (MIC) considered clinically meaningful (3.7-4.5 MIDAS points), the goal was to obtain a 5-point improvement in MIDAS scores after 3 months of REN use. To detect this improvement with a 2-tailed paired samples t test, an α of 5%, 80% power, and an assumed SD of 1, the minimum required sample size was calculated as 384. The CED end date was set based on this predefined sample size and expected enrollment rate.
Outcomes
Primary end point. The primary outcome was the change in quality of life measured by the MIDAS questionnaire, comparing the 3 months before (baseline) and after starting treatment with the REN wearable. MIDAS assesses the number of days affected by migraine in work, household, and social activities, offering a comprehensive disability meter. Its simplicity, reliability, and validity make it accessible to both patients and health care providers, enhancing patient-physician communication and treatment planning. Additionally, MIDAS can track changes over time, making it valuable for evaluating the effectiveness of therapeutic interventions, including pharmacological medications for the acute treatment of migraine.
Secondary end points. The secondary outcomes were as follows: (1) prospective 2-hour efficacy, which was measured according to the International Headache Society guidelines.36 Treatments for which both baseline and 2-hour posttreatment pain or disability were reported were termed evaluable and used for analysis. As patients voluntarily recorded these parameters, the number of evaluable treatments differed for each efficacy metric. The proportion of patients consistently achieving 1 of the following 4 efficacy metrics in at least 50% of their evaluable treatments when REN was used as a stand-alone therapy (ie, no medication intake was reported 2 hours from starting a REN treatment) was calculated. All changes were measured from baseline to 2 hours post treatment: (a) pain relief: decrease in headache severity from moderate or severe to mild or no pain; (b) pain freedom: decrease in headache severity from mild, moderate, or severe to no pain; (c) functional disability relief: improvement of at least 1 grade of functional disability; and (d) functional disability freedom: no functional disability following any level of functional disability; (2) evaluation: the proportion of patients reporting satisfaction, effectiveness, and tolerability as measured by the online TSTQ using a 1 to 5 Likert scale. A positive result is defined as a score of 5 (completely satisfied) or 4 (slightly satisfied) per domain; (3) safety: assessed using an adverse event tracking system in the Nerivio application and by flagging any adverse event complaints to the customer support call center. Additionally, it was assessed by an online questionnaire administered 3 months post treatment initiation; and (5) utilization: (a) the proportion of participants who refilled their Nerivio device at least once and (b) the mean number of REN devices filled per patient per year. Because the CED enrolled patients on a rolling basis, the duration of participation varied among patients when the analysis was performed. The total number of devices filled per patient during their respective participation duration was divided by the number of days they participated in the study and multiplied by 365 to estimate the annual fill rate.
Data Analysis
Demographic and clinical characteristics are presented as mean (SD) for continuous variables and as numbers and percentages for nominal variables. A 2-tailed paired t test with a statistical significance of P<.05 was used for the primary end point. Secondary end points are presented as frequency and percentages for nominal variables and mean (SD) for continuous variables. Data analysis was conducted using Excel 365 (Microsoft).
RESULTS
Participants
Between November 2022 and February 2024, 381 patients with migraine (99.2% of the target sample size) were prescribed the REN wearable and used it for at least 3 months. Participants were aged 13 to 80 years (mean [SD], 40.5 [13.2] years), and 91.1% were female (n=347) (Table 1). Of those with a reported International Statistical Classification of Diseases, Tenth Revision (ICD-10) code (n=162), 56.2% were diagnosed with chronic migraine and 43.8% with episodic migraine, and 4.3% were also diagnosed with MOH. Migraine with aura was present in 29.7% of patients. Midstudy, the indication for REN was expanded to include migraine prevention treatment. Before expansion, all patients (n=251) were prescribed REN for acute treatment. Post expansion, most patients (70.4%) were prescribed REN for prevention or dual use. Prescription data from 23 participants were missing. Overall, most patients (83.2%; 298 of 358) were prescribed REN for acute treatment, and the remaining 16.8% were prescribed REN for migraine prevention or dual use (5.3% and 11.5%, respectively). All adults enrolled in the CED had experienced failure of least 1 acute migraine medication, most commonly nonsteroidal anti-inflammatory drugs (10.8%), triptans (11.5%), or both (74.3%). For some (29.7%), gepants or other second line nongeneric medications had failed. Patients completed a total of 7854 REN treatments, with a mean (SD) of 20.6 (26.8) treatments per patient. The distribution of the number of REN treatments per patient is listed in Table 1.
Primary End Point
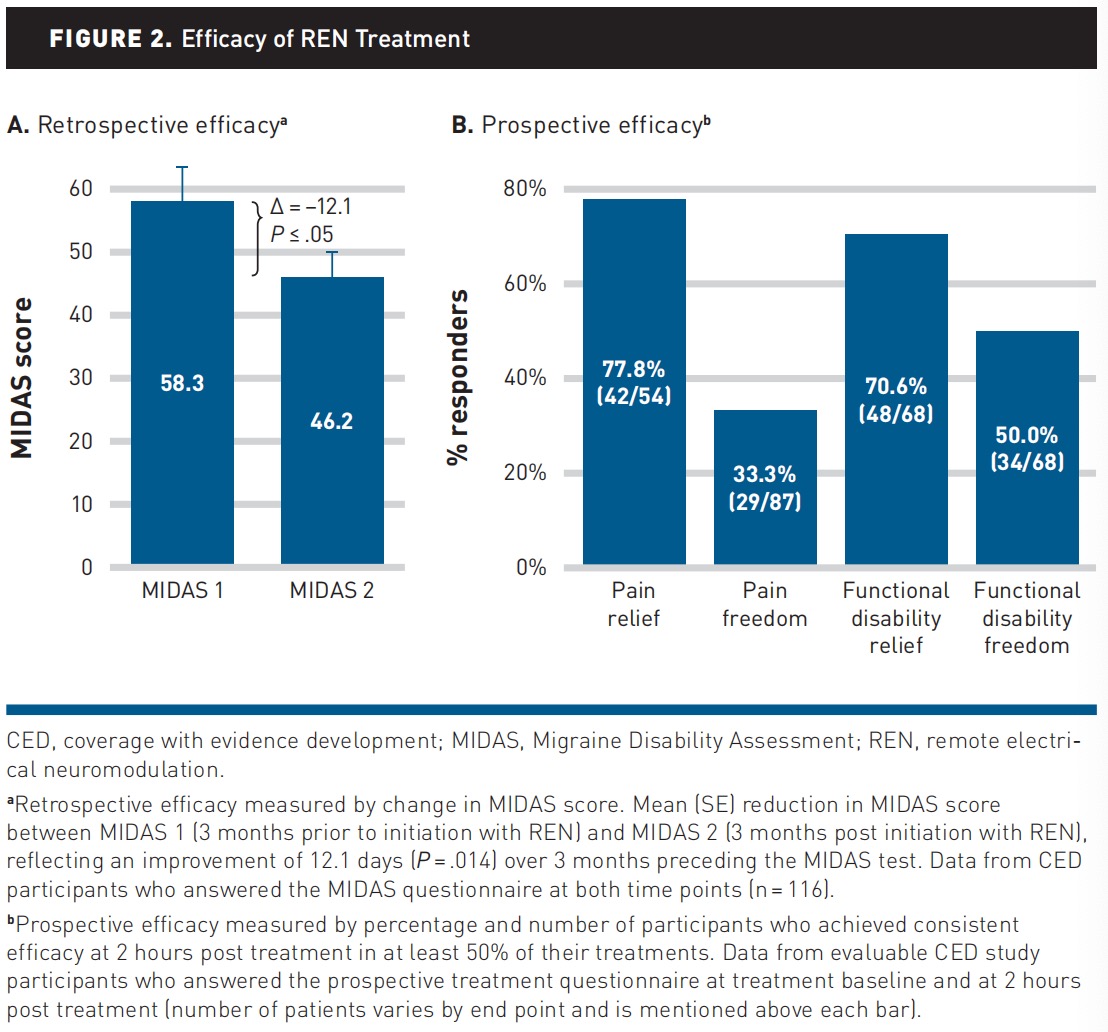
The primary end point analysis included 116 (30.4%) participants with baseline and 3-month MIDAS scores. Mean (SD) baseline MIDAS score was 58.3 (59.0), indicating severe disability (grade 4) due to migraine.29 Following 3 months of treatment, the mean (SD) MIDAS score decreased by –12.1 (51.8) MIDAS points to 46.2 (44.1), a statistically significant (P =.014, paired t test) and clinically meaningful reduction (Figure 2 [A]). Half (58 of 116) of these participants achieved a clinically meaningful improvement with a reduction of 4.5 points or more.
Secondary End Points
Prospective efficacy. Analyses of consistent efficacy (in ≥50% of treatments per patient) showed that 77.8% (42 of 54) of participants experienced pain relief, 33.3% (29 of 87) experienced pain freedom, 70.6% (48 of 68) experienced functional disability relief, and 50.0% (34 of 68) experienced functional disability freedom (Figure 2 [B]).
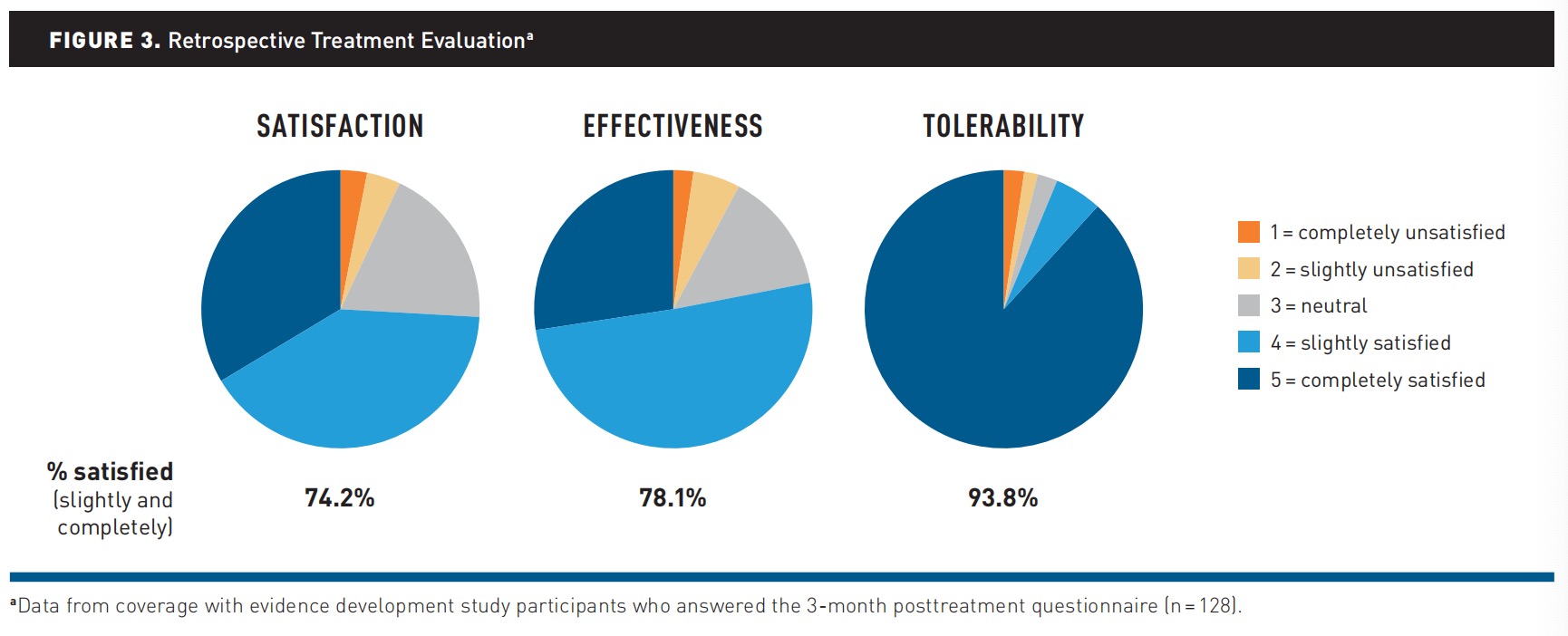
Evaluation. Of those who provided feedback (33.6%; 128 of 381) via the TSTQ, 74.2% (95 of 128) reported satisfaction, 78.1% (100 of 128) reported treatment effectiveness, and 93.8% (120 of 128) reported tolerability (Figure 3).
Safety. Of 381 study participants, only 1 patient (0.2%) reported a minor adverse event (muscle twitching) to the customer support call center. Following this report, the patient continued using REN. Two of the 128 (1.56%) TSTQ responders reported adverse effects; neither of them contacted the investigators or the customer call center, suggesting that their adverse effects were not serious enough to require medical attention.
Utilization. A total of 39.1% (149 of 381) of study participants refilled their REN prescription at least once. Extrapolated mean (SD) annual device use per patient was 4.0 (3.1).
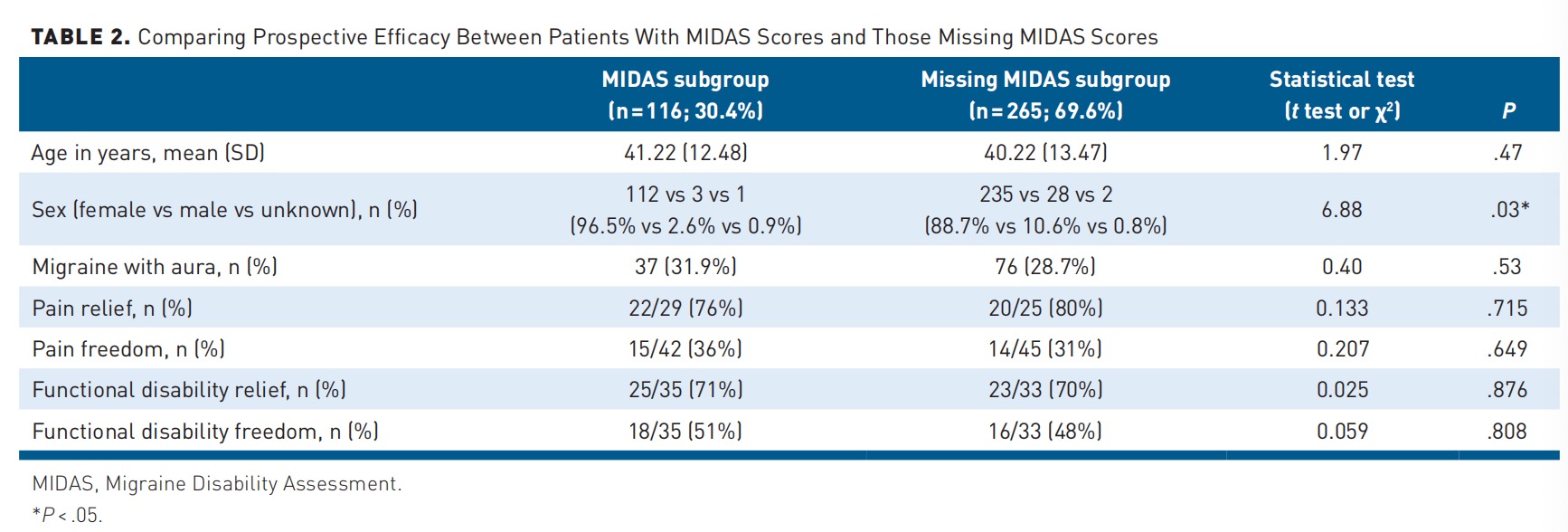
Missing Data Analysis
Given that 69.6% of the study cohort lacked primary end point data, a subanalysis compared baseline characteristics and secondary efficacy end points of participants with and without MIDAS scores.
Age and aura prevalence were similar between groups, although more female participants completed the MIDAS questionnaire. No differences in any prospective efficacy end points were found among the subgroups (Table 2).
DISCUSSION
This CED study by Highmark evaluated the efficacy, utilization, safety, and cost-effectiveness of covering the REN wearable treatment to inform coverage decisions and set clinical practice guidelines for this device as a standard-of-care covered migraine treatment.
The primary end point showed a statistically significant mean (SD) reduction of 12.1 (51.8) points (P =.014) in MIDAS score after 3 months of REN use. This improvement is larger than that reported for pharmacological medications, such as combining sumatriptan with naproxen as abortive treatment, which produced a 6.1-point reduction after 3 months. Moreover, this result exceeds the MIC considered clinically meaningful by Lipton et al and Carvalho et al of an increase/decrease of 3.7 days of migraine-related disability or 4.5 MIDAS points. The mean reduction of 12.1 MIDAS points represents an improvement approximately 3-fold (times 3.3 or 2.7, respectively) greater than the MIC set in earlier studies, reflecting a mean monthly improvement of 4.0 days of migraine-related disability. This translates to patients gaining 4 monthly workdays, school days, or general days of productivity that would have otherwise been lost or impaired due to migraine, if not for REN treatment.
The secondary end points further assessed REN treatment efficacy by evaluating patient-reported prospective change in pain severity and functional disability from treatment onset to 2 hours post treatment. To isolate the effect of REN as a standalone therapy, the analysis included only evaluable treatments when no other medications were taken within the 2-hour assessment window. First, of the participants with pre- and posttreatment data, more than three-fourths (77.8%) reported pain relief, and one-third (33.3%) reported pain freedom. These results replicate findings from the RCT of REN10 and subsequent clinical trials and real-world studies. Second, these figures are equivalent or superior to those achieved by most traditional acute migraine treatments in the triptan family (range, 31.1%-74%), which are commonly prescribed as first-line treatments and are covered by US health insurance companies. Third, the reported REN efficacy results are comparable to or surpass those of new medications, such as oral CGRP receptor antagonists and ditans, commonly covered by US payers as second-line treatments, in which 2-hour pain relief and freedom are reported in 58% to 65% and 20% to 39% of patients, respectively. In addition to pain data, the REN smartphone application allowed patients to report prospective functional disability data per treatment. More than two-thirds of the participants (70.6%) experienced relief from migraine-related functional disability 2 hours post treatment, and 50.0% experienced a return to normal function. This proportion is markedly higher than reports for new drugs such as oral CGRP receptor antagonists and ditans, yielding post–2 hours disability freedom in approximately 32% to 43% and 37.0% of patients, respectively, and freedom from most bothersome symptom in 35% to 38% and 48.7% of patients, respectively.52 Despite the different study designs and literature comparison (rather than head-to-head), using the same 2-hour end points lends clinical relevance to these indirect comparisons. These data suggest the REN device can lead to quick and effective relief or resolution of migraine headaches and debilitating associated symptoms, potentially decreasing rates of absenteeism (missed workdays), presenteeism (reduced productivity at work), and health care resource utilization to a similar or greater extent than common migraine treatments.
A subset of patients in this CED study (n=128; 33.6%) completed the TSTQ after using REN for at least 3 months. Compared with data from the DMKG Headache Registry on acute migraine medications including triptans and common OTC analgesics, the REN wearable showed similar or higher rates in each of the 3 outcomes: retrospective treatment efficacy, reported by 78.1% of CED participants vs 75.5% of triptan patients and 43.7% of OTC patients; tolerability, reported by 93.8% of CED participants vs 68.2% and 76.7% of triptan and OTC patients, respectively; and overall satisfaction, reported by 74.2% of CED participants vs 65.4% of triptan patients and 46.8% of OTC patients. These data indicate that patients perceive the REN device to be at least as effective as triptans and more effective than OTC analgesics, with significantly higher tolerability and overall satisfaction.
Unlike pharmacological treatments, which often rely on patient reports, this study leveraged the REN wearable app to precisely monitor participants’ adherence and utilization. This revealed a mean (SD) annual usage of 4.0 (3.1) devices per patient informing the payer’s economic calculations. Additionally, the mean reduction of 4.0 migraine days per month revealed by the MIDAS questionnaire may suggest potential cost savings through decreased physician appointments, urgent care/emergency department visits, and medication intake. Lastly, the low incidence of device-related adverse events, none of which were serious or severe, aligns with previous research supporting REN’s favorable safety profile. This, in turn, may reduce health care utilization by mitigating the risk of MOH, a known driver of increased medical care.
Limitations
This CED program offers valuable data on REN’s efficacy and patient experience in a real-world clinical setting. Nevertheless, there are several limitations to this study. Initially, the study focused solely on acute migraine treatment. A few months into the program, the indication was expanded to include prevention use as well, but study end points were already set and could not assess prevention efficacy directly, leaving room for future research to explore the long-term effectiveness and potential cost savings associated with REN dual use. Second, questionnaire completion (MIDAS, TSTQ) was not mandatory, although the response rate of one-third was high for real-world health surveys, and missing data analyses show no differences in prospective efficacy between 3-month MIDAS responders and nonresponders. Additionally, the fact that more female participants answered the MIDAS questionnaire aligns with global patterns of surveys.56,57 Last, a full health economics analysis was not included in the current analyses, although ample data, mentioned earlier, supported a positive coverage decision.
CONCLUSIONS
The results of this CED study demonstrated statistically significant and clinically meaningful benefits to patients with migraine using the REN wearable in real-world settings. Given the need for safe and effective migraine treatments, the results highlight the role REN should play as a covered, and thus more accessible, therapy. Although prescribing decisions remain with educated providers, health plans should support this professional decision with adequate coverage.
