Comparison of remote electrical neuromodulation (REN) and standard-care medications for acute treatment of migraine in adolescents: a post-hoc analysis
Andrew D. Hershey et al, Pain Medicine, 2021
Abstract
Objective: There is an unmet need for new efficacious, well-tolerated, acute treatments for migraine in adolescents. Remote electrical neuromodulation (REN) is a novel, non- pharmacological treatment, that provides significant symptom relief with good tolerability. The current post-hoc analysis compared the efficacy of REN to that of standard-care medications, for the acute treatment of migraine in adolescents.
Design: Within-participant post-hoc analysis of data from a clinical trial.
Setting: Data from a clinical trial.
Subjects: Data from 35 adolescent participants was analyzed.
Methods: Efficacy was compared between a run-in phase in which attacks were treated with standard-care medications (triptans or over-the-counter medications), and an intervention phase in which attacks were treated with REN. Efficacy was compared within-participant using McNemar’s test, at four endpoints (two hours post-treatment): single-treatment pain freedom and pain relief, and consistency of pain freedom and pain relief (defined as response in at least 50% of the available first four treatments).
Results: At two hours post-treatment, pain freedom was achieved by 37.1% of the participants with REN, vs. 8.6% of the participants with medications (p=0.004). Pain relief was achieved by 71.4% with REN, vs. 57.1% with medications (p=0.225). Consistency of pain freedom was achieved by 40% with REN, vs. 8.6% with medications (p<0.001). Consistency of pain relief was achieved by 80.0% with REN, vs. 57.2% with medications (p=0.033)
Conclusions: Our results suggest that REN may have higher efficacy than certain standard-care medications for the acute treatment of migraine in adolescents. A larger scale, blinded, comparative effectiveness and tolerability study is needed.
Keywords
Migraine, Headache, Adolescents, Treatment, Nerivio, REN
Introduction
Migraine is a prevalent and debilitating disease, affecting approximately 9% of children and adolescents worldwide. Migraine prevalence increases during adolescence, and is associated with poorer academic performance, reduced school attendance, and a negative effect on social interactions and quality of life. Current acute treatments for adolescents with migraine are mostly pharmacological. These treatments may cause side effects, and their frequent ruse may potentially lead to medication overuse headache and migraine chronification to the point of chronic migraine. Additionally, their efficacy may be variable or inadequate. Thus, there is a great unmet need for new safe and effective acute treatments for adolescents with migraine headaches.Recently, several non-pharmacological, non-invasive neuromodulatory approaches were introduced for the treatment of migraine. Remote Electrical Neuromodulation (REN) stimulates nociceptive nerve fibers in the upper arm to activate an endogenous descending pain inhibition mechanism termed Conditioned Pain Modulation (CPM), see figure 1. Arecent open-label multicenter study has demonstrated that REN provides clinically meaningful relief of migraine pain in adolescents. That study joins previous efficacy studies that have demonstrated that REN is safe and clinically beneficial for the acute treatment of the attacks of migraine in adults with both episodic and chronic migraine. Additionally, a recent meta- analysis found REN to be the only migraine neuromodulation intervention for which there is sufficient high-quality research, and thus the only one for which efficacy was well established. The current post hoc analysis aims to compare the efficacy of REN for the acute treatment of attacks in adolescents with migraine, to that of contemporary standard-care medications; oraltriptans and over the counter (OTC) analgesic medications. For that purpose, a within-participant, post-hoc analysis was performed, based on data from the aforementioned clinical trial (ClinicalTrials.gov NCT04089761), which examined REN’s efficacy for treating attacks of migraine in adolescents.
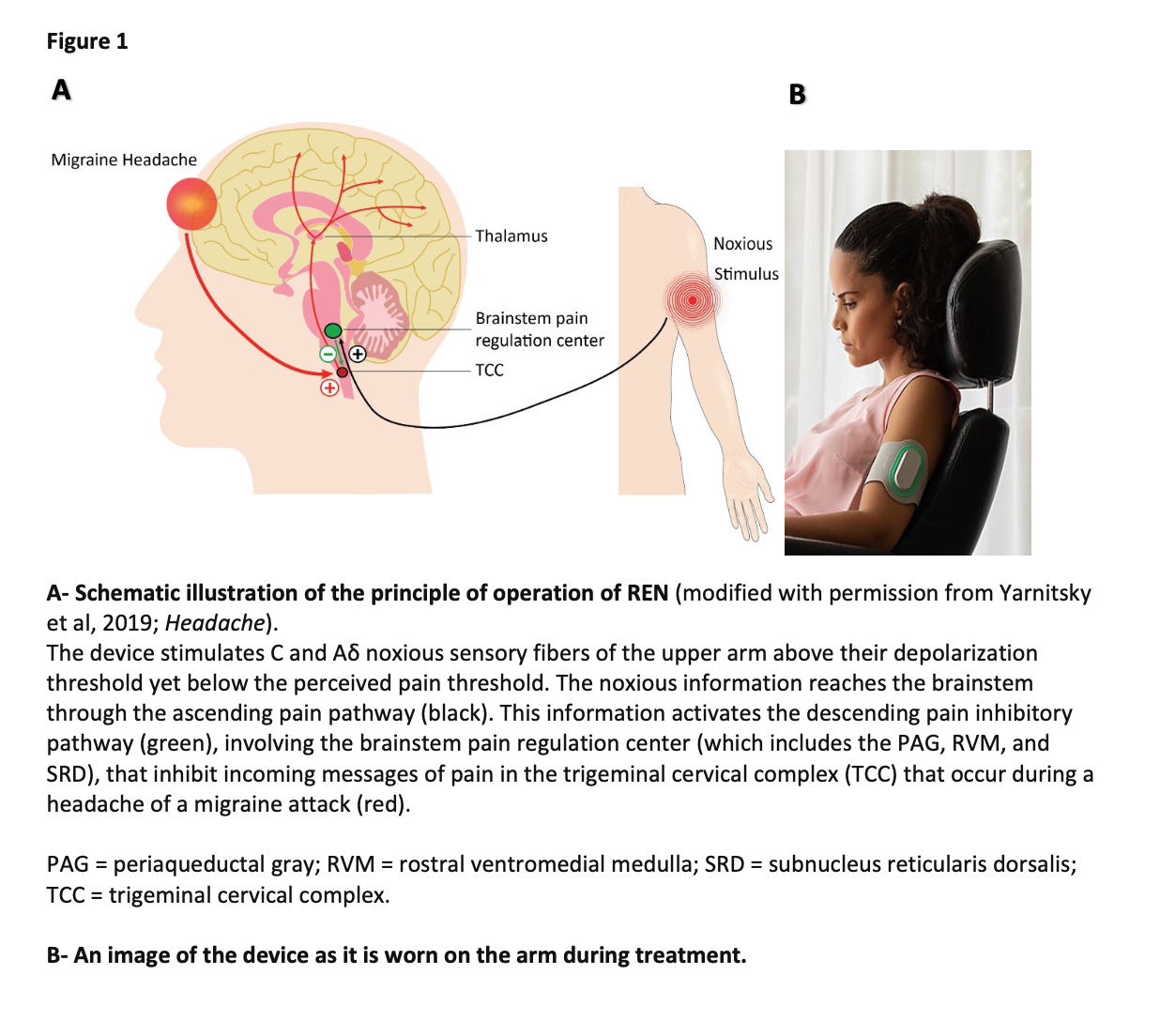
Methods
Dataset
This post-hoc analysis was conducted on data from an open-label, multi-center clinical trial, which evaluated the safety and efficacy of REN as an acute migraine attack treatment in adolescents (15). The first phase of that study was a four-week run-in phase (“medication phase”). In that phase, participants were instructed to properly treat their migraine attacks with their usual over the counter or prescription medications. Participants were asked to report their symptoms immediately before the treatment, two hours post treatment, and 24 hours post treatment, for each treatment session (using an electronic diary). In the REN treatment phase (“REN phase”), participants received a REN device (Nerivio®, Theranica Bio-electronics Ltd., Israel) and were instructed to treat their attacks only with the device, and refrain from any acute medications for at least 2 hours, while continuing to report their symptoms and treatment results (as in the medication phase). Of the 39 participants who used the REN to treat an attack in the REN phase, 35 used migraine medications during the run-in phase. The electronic-diary reports of these 35 participants (from both phases) comprise the dataset for the current analysis. For all pain reports in the electronic diary a four-level scale of pain intensity was used: no pain, mild, moderate or severe. All analyzed treatments were preceded by at least 24 hours of headache freedom (as reported by participants).
The current study is a post-hoc analysis of a clinical study (ClinicalTrials.gov NCT04089761).
The clinical study was approved by Western Institutional Review Board (WIRB; approval number 20192678) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants prior to the start of the study.
Endpoints
Four efficacy endpoints were tested: pain freedom and pain relief (severe to mild or no pain, or moderate to no pain – 2 step improvement) following a single treatment, and pain freedom and pain relief consistency across treatments. All endpoints refer to two hours post treatment. For the single treatment endpoints, the analyzed test-treatment for REN was defined as the first REN treatment following a training treatment, and the test-treatment for medications was defined as the first treatment in the run-in phase in which medication was used. For the consistency endpoints, consistent response was defined as response (pain freedom or relief) in at least 50% of the first four treatments. All participants had completed four evaluable REN treatments. The number of medication treatments varied between participants, and consistency was calculated based on all available data (up to four treatments) for each participant.
Data analysis
Efficacy outcomes were calculated using McNemar’s test, comparing contingency data for matched pairs nominal (yes/no) values. The odds ratio (O.R.) between the two phases, and the estimated differences between the paired proportion were calculated using Fisher’s confidence interval (C.I.). All statistical tests were two-tailed, with statistical significance set to p<0.05. No adjustments were made for multiple comparisons. Tests were conducted using SPSS Statistics
Results
Participants
Of the 35 participants in this post-hoc analysis, 26 (74.2%) were girls. The average age was 15.6 ±1.8 years old. Thirty-one participants (88.5%) were White, three (8.6%) were African American, and one (2.9%) was Hispanic. Five of the participants (14.2%) had chronic migraine, while the remainder had less than 15 headache days per month - 30 (85.8%). Twenty participants (57.1%) were on a stable preventive medication for migraine prior to enrollment. During the medication phase, 8 participants (22.8%) used oral triptans only (5 rizatriptan, 1 eletriptan, 1 zolmitriptan, 1 sumatriptan), 2 participants (5.7%) alternated between oral triptans and OTC analgesics, and 26 participants (71.4%) used OTC analgesics only (11 ibuprofen, 7 naproxen sodium, 5 acetaminophen, 3 acetaminophen-aspirin-caffeine combination).
Baseline characteristics of migraine attacks
The baseline characteristics of the attacks in the medication phase and the REN phase are presented in Table 1. The characteristics of the test treatment in the two phases were not statistically different with respect to pain severity, photophobia, phonophobia, and nausea. The characteristics of the treated attacks in both phases were similar to those reported in previous studies of migraine and are consistent with those of the target population .
Efficacy results
Single treatment:
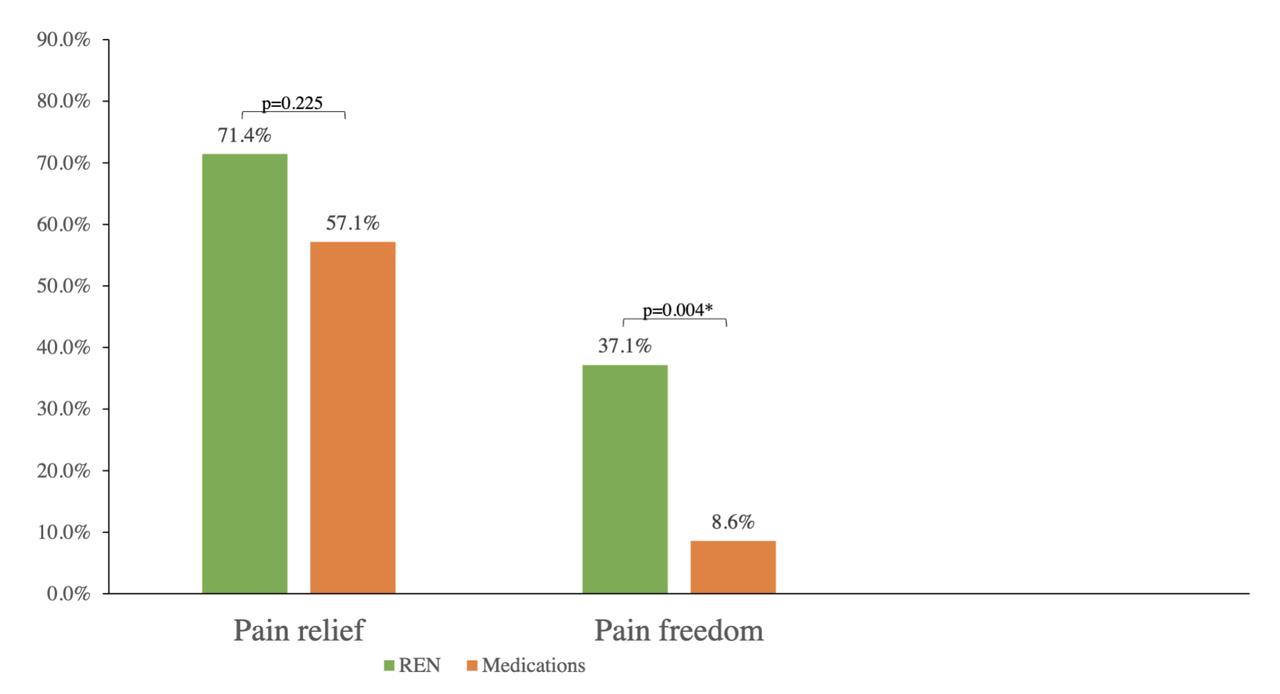
Pain freedom in REN phase was reported by 37.1% (13/35) of the participants, vs. 8.6% (3/35) in the medication phase, with a statistically significant difference: p=0.004 (O.R.=11.0, 95% C.I.1.60-473).Pain relief in the REN phase was reported by 71.4% (25/35) of the participants, vs. 57.1% (20/35) in the medication phase, p=0.225 (O.R.=1.83, 95% C.I. 0.62-6.04).
The single-treatment results are depicted in Figure 2.
Consistent efficacy:
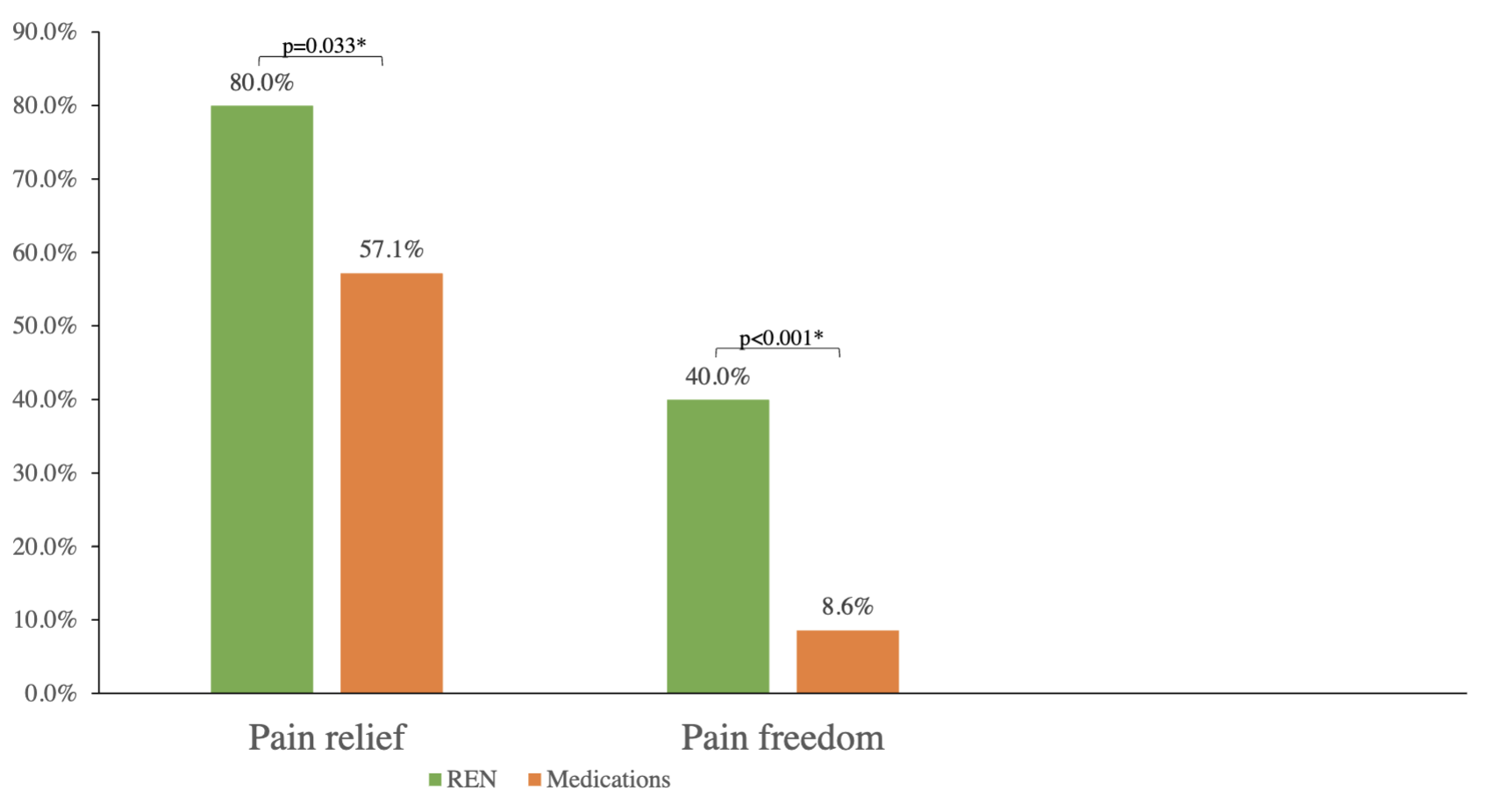
Consistent pain freedom in REN phase was reported by 40.0% (14/35) of the participants, vs. 8.6% (3/35) in the medication phase, with a statistically significant difference: p<0.001 (proportion difference=0.314, 0.144-0.473). O.R. was not applicable due to zero participants in the group that achieved pain freedom with medications but not with REN, thus the paired proportion difference was estimated instead.
Consistent pain relief in the REN phase was reported by 80.0% (28/35) of the participants, vs. 57.1% (20/35) in the medication phase, with a statistically significant difference: p=0.033 (O.R.=3.67, 95% C.I. 0.97-20.47).
The consistency results are depicted in Figure 3.
Response rates by medication class
Response rates were sub-analyzed by medication class. Statistical comparison was not performed to due sample size limitation of the sub-groups, and response rates are presented for reporting purposes only. Twenty-six participants used OTC analgesic medications, of whom 38.4% (10/26) achieved pain freedom with REN, vs. 11.5 % (3/26) with medications. Pain relief was achieved by 76.9% (20/26) with REN, vs. 65.3% (17/26) with medications. Nine participants used oral triptans, of whom 33.3% (3/9) achieved pain freedom with REN, vs. 0% (0/9) with medications. Pain relief
Adverse events
Eight participants (22.9%) reported adverse events in the REN phase. There was one mild device-related adverse event reported (2.9%), in which a temporary feeling of pain in the arm was felt but resolved after the treatment without requiring intervention. The other adverse events were deemed unrelated to the device: common cold, chest congestion, influenza leg pain, streptococcus pharyngitis, upper respiratory infection, and worsened migraine (1). There were no device-related serious adverse events and none of the participants withdrew from the study due to device-related adverse events.
Discussion
The results of the current analysis suggest that REN may be more effective for the acute treatment of migraine in adolescents compared to certain standard-care medications. Specifically, REN was found to be superior to the tested medications (oral triptans and OTC analgesic medications) in pain freedom following a single treatment, pain freedom consistency across treatments, and pain relief consistency across treatments. REN and medications were similarly effective for pain relief following a single treatment.
This analysis joins previous findings from a similar post-hoc analysis in adults with migraine . Taken together, the current preliminary results suggest that REN may provide a non- pharmacological treatment option for adolescents with migraine, which may be more effective than certain common pharmacological treatments, with a favorable side effect profile. The pain freedom rate with medications was low in comparison to the few previous studies that tested migraine pain freedom as a primary endpoint in adolescents for oral triptans (e.g. - age range 12-17) and ibuprofen- age range 6-12). This could potentially raise the question of whether the sample tested in the current study did not represent the population of adolescents with migraine (e.g., patients seeking alternate treatment due to insufficient response to medications). However, unlike pain freedom, the rate of pain relief with medications in our study is comparable to results reported in those very same studies suggesting that that the samples are otherwise comparable in terms of treatment response. Additionally, none of the participants took a triptan-NSAID (Nonsteroidal anti-inflammatory drugs) combination, known to have a relatively high response rate.
The current study has several limitations. First, the small sample size limits the generalization of the results. While corroboration is needed in a larger sample, statistically significant differences were found even within the limited sample size, suggesting a true difference. The sample size also curtails a sub-analysis of the efficacy by medication type. Indeed, no conclusions can be drawn from the current analysis regarding the specific efficacy comparison with either triptans or OTC alone. However, the comparability of the medication classes is supported by a review indicating that in a clinical trial setting, the efficacy of specific and non-specific migraine acute mediations (including NSAIDs, acetaminophen, and triptans) is comparable . Furthermore, we provide response rates by medication class that show that in all instances REN had a (qualitatively) higher number of participants that achieved response. Finally, consistency analysis of the medication data was performed on naturalistic observational data (i.e., participants were instructed to continue their usual care during the run-in phase). It thus includes in some cases different pharmacological treatments by the same participant, as well as instances where less than four pharmacological treatments were available for the consistency analysis (in which case consistency was calculated based on all available treatments). While introducing variability into the data, these individual differences may better reflect real-world migraine management, which varies across attacks. It should also be noted that different treatment modalities have been shown to have a different rates of placebo response (33), and in the case of pharmaceuticals and medical device this could also not be ruled out.
Conclusion
The current post-hoc analysis of data from a clinical trial provides preliminary indication that REN may be as effective, if not more effective than certain standard-care medications in adolescents with migraine. Combined with its low rate of side effects, REN may thus offer a novel alternative for current pharmacological treatments for teenagers with migraine.
Acknowledgements
We thank the participants in this trial and the investigators and site personnel. We also acknowledge Lonnie Zeltzer, MD, PhD, who served as the chair of the DSMB in the study, Dr. Nira Morag for statistical consultation and analyses, and FlaskData.IO (Modi’in, Israel) for the electronic data collection system used in this study.
