Nonpainful remote electrical stimulation alleviates episodic migraine pain
David Yarnitsky et al, American Academy of Neurology, 2017
ABSTRACT
Objective: To evaluate the efficacy of remote nonpainful electrical upper arm skin stimulation in reducing migraine attack pain.
Methods: This is a prospective, double-blinded, randomized, crossover, sham controlled trial. Migraineurs applied skin electrodes to the upper arm soon after attack onset for 20 minutes, at various pulse widths, and refrained from medications for 2 hours. Patients were asked to use the device for up to 20 attacks.
Results: In 71 patients (299 treatments) with evaluable data, 50% pain reduction was obtained for 64% of participants based on best of 200-ms, 150-ms, and 100-ms pulse width stimuli per individual vs 26% for sham stimuli. Greater pain reduction was found for active stimulation vs placebo; for those starting at severe or moderate pain, reduction (1) to mild or no pain occurred in 58% (25/43) of participants (66/134 treatments) for the 200-ms stimulation protocol and 24% (4/17; 8/29 treatments) for placebo (p 5 0.02), and (2) to no pain occurred in 30% (13/43) of participants (37/134 treatments) and 6% (1/17; 5/29 treatments), respectively (p 5 0.004). Earlier application of the treatment, within 20 minutes of attack onset, yielded better results: 46.7% pain reduction as opposed to 24.9% reduction when started later (p 5 0.02).
Conclusion: Nonpainful remote skin stimulation can significantly reduce migraine pain, especially when applied early in an attack. This is presumably by activating descending inhibition pathways via the conditioned pain modulation effect. This treatment may be proposed as an attractive nonpharmacologic, easy to use, adverse event free, and inexpensive tool to reduce migraine pain.
GLOSSARY
ANOVA 5 analysis of variance; CPM 5 conditioned pain modulation; ITT 5 intention-to-treat; NNT 5 number needed to treat; NPS 5 Numeric Pain Scale; ONS 5 occipital nerve stimulation.
In this study, we stimulated remotely in order to relieve migraine pain soon after onset. Our rationale is activation of pain inhibitory centers, via the conditioned pain modulation (CPM) effect; remote noxious stimuli can exert a generalized analgesic effect. This is by the descending analgesia tracts originating at brainstem centers and terminating at spinal, including cervical trigeminal, nuclei. Since use of pain to inhibit another pain is not clinically appealing, we use nonpainful conditioning; we and others have shown that robust nonpainful conditioning stimuli are sufficient in many cases to induce pain inhibition. Presumably, the threshold for activation of the inhibitory pain control system is lower than that of pain perception. The current study aims to peruse this gap by inducing generalized pain inhibition by well-perceived but not painful remote electrical stimulation.
This approach seems well-suited for migraine attacks since (1) in the beginning of a migraine episode, pain is usually relatively low, and sensitization has not yet taken place, thus the limited magnitude of the effect has a potential for clinical efficacy; (2) pain inhibiting pain has a very short aftereffect, so continuous pain syndromes will require continuous use of the device, while episodic migraine self terminates, allowing short duration use; and (3) an electrode on the upper arm has lower visibility and is more convenient than one on the head. Our hypothesis is that application of remote electrical stimulation at an intensity lower than pain threshold will generate sufficient inhibitory effect to abort, or at least substantially reduce, a migraine attack at its onset, when pain level and sensitization are still low. Given the episodic nature of migraine headache, such pain relief will be highly beneficial for patients.
METHODS
This is a prospective, double-blind, randomized, crossover, sham-controlled trial conducted in the Neurology Department of Rambam/Technion (Haifa, Israel). Standard protocol approvals, registrations, and patient consents. The study was approved by the Rambam Ethics Committee. Standard written informed consent was obtained from all participants. The study was registered as NCT02453399.
Participants. Eighty-six episodic migraineurs with and without aura who met the International Headache Society criteria and had 2–8 attacks per month without preventive medications for at least 2 months were recruited. Exclusion criteria were as follows: (1) other significant pain problem such as cancer pain, fibromyalgia, or other head or facial disorder; (2) severe cardiac or cerebrovas cular disease; (3) uncontrolled high blood pressure; (4) implanted electrical or neurostimulation devices; (5) epilepsy; (6) use of cannabis; (7) chronic migraine (8) head or neck nerve block within the last 2 months; (9) Botox injections within the last 6 months; (10) pregnant or planning pregnancy during the study period, or is in childbearing years and unwilling to use an accepted form of birth control (11) participation in another migraine clinical study; and (12) lack of sufficient cognitive or motor skills needed to operate android cell phone.
Treatment and randomization. The stimulating device (Nerivio Migra, Theranica Ltd., Netanya, Israel) consists of a pair of rubber electrodes mounted on an armband with a power source, controlled by the patient’s smartphone, via a custom made application. Five 20-minute-long stimulation protocols were programmed in each unit; 4 active programs at 80–120 Hz, with pulse widths of 200 (P200), 150 (P150), 100 (P100), and 50 (P50) ms, and 1 placebo stimulation protocol (P0) at 0.1 Hz frequency with 45-ms-long pulses. We used several pulse widths in order to explore the stimulus-response relationship of the effect, as well as to identify the pulse width generating best efficacy. Stimuli were given at random sequence at the following distribution: P0 and P200: probability of 1/3 each; P50, P100, P150: probability of 1/9 each. Higher probabilities were selected for placebo and P200 as the above were hypothesized to be of primary interest for comparison; intermediate programs were included in order to provide treatment alternatives in the case when P200 is not tolerable to some participants, as well as to explore dose response effect. Both patients and study personnel were blinded to the order of individual treatments.
Use of the device and application were demonstrated in a training session. During the study period, participants were requested to mount the electrodes on their right or left arm, per their choice and regardless of the side of migraine pain, and activate them, for 20 minutes, as soon as possible after attack onset. They were instructed to adjust the stimulus, via their smartphone, to a well-perceived, but not painful level, and readjust along the stimulation period. Patients were asked to refrain from use of medications for 2 hours starting at stimulation onset. They were requested to use the device for up to 20 migraine attacks.
Data reporting and collection. Pain levels were self-reported via the smartphone application at onset and 10, 20, and 120 minutes after stimulation onset. Numeric Pain Scale (NPS) 0–10 was displayed as a slider control with numeric annotations. The participants also reported time from attack onset to treatment onset, their experience with the treatment itself, whether rescue medication was used within the 2 hours, as well as additional comments. Repeated treatments in the course of an attack were permitted. Reported data were automatically transferred to a centralized database where they were stored and archived until unlocked and analyzed. Inflow of data was regularly monitored by study personnel, but exposed only to a limited number of personnel, those who did not have any contact with the participating patients.
Every patient participated in 2 follow-up phone interviews, 2 weeks and 2 months into the experiment, asking for feedback regarding treatment perception, adverse effects, use of migraine medications, and overall usability of the method. In addition, participants were instructed to report any adverse events to the study coordinator within 48 hours of their occurrence. All adverse effects were recorded in case report forms and followed through by study personnel.
Outcome measures. Primary endpoints were calculated based on all pain levels at the beginning of treatments. One primary endpoint was percentage of responders to all stimuli: percentage of patients reporting pain decrease of at least 50% at 2 hours post treatment, in at least 50% of completed treatments. The other was the relative pain reduction by NPS at 2 hours posttreatment as percentage of pretreatment pain. This was calculated per each type of stimulation.
In addition, in order to provide a basis for comparison to the major pharmacologic randomized controlled trials in migraine treatment (for review, see reference 11), we report results in terms of pain grades based on levels of moderate and severe pain at beginning of treatment; NPS data were converted into pain grades according to the following scheme: 0, 1, no pain; 2, 3, mild pain; 4–6, moderate pain; 7–10, severe pain.We calculated percent of participants reporting pain reduction from moderate or severe to (1) mild or no pain; and (2) to no pain, both at 2 hours posttreatment. These results are also presented in terms of number needed to treat (NNT).
We also followed treatment effect as a function of time between attack onset and treatment onset. For the purpose of this analysis, only first treatments within every treated attack were considered.
At the end of each treatment, participants were asked to rate their treatment perception selecting one of the following options: painful, unpleasant, pleasant, very pleasant. A similar scale was used at end of the study. Their assessment on the overall use of migraine medications during the study period was estimated in a poststudy questionnaire as one of the following: 0 = more, 1 = same, 2 = less. The burden of treatment was assessed as one of the following: 0 = very burdensome, 1 = slightly burdensome, 2 = neutral, 3 = not at all. Ease of device and application use were evaluated by the subject as one of the following: 0 = very complicated, 1 = complicated, 2 = neutral, 3 = easy, 4 = very easy.
Statistical analysis. Statistical analysis was performed on modified intention-to-treat (ITT) data. Multiple imputation method was used to generate the ITT dataset, which contained all participants who successfully performed at least one treatment session. Imputations were generated from distributions empirically fitted to available data for each metric and each individual treatment program. Multiple imputed datasets were created using independent realizations of the corresponding missing points via random number generators. Results from all realizations were statistically analyzed and further averaged.
McNemar test was used for comparison of matching responder rates. For analysis of responder rates, placebo results were evaluated vs best of the active programs. Tests were 2-tailed, with p , 0.05 considered statistically significant. Bonferroni correction was applied to compensate for multiple comparisons. Analysis of variance (ANOVA) was carried out for comparison of treatment efficacy between individual programs.
This interventional study provides Class III evidence that nonpainful remote electrical stimulation is efficient in alleviating episodic migraine pain in regards to the 2 primary endpoints.
RESULTS
Patients. The study was performed between June 2015 and March 2016. A total of 86 participants were handed the Nerivio Migra devices. A summary of participants’ demographic characteristics is presented in table 1. Seventy-two participants successfully treated at least one migraine attack; the rest either did not treat their attacks per protocol or failed to provide complete feedback. One participant was excluded from statistical analysis due to repeated use of rescue medications concurrently with the electrostimulation treatments. Data of 71 participants, 949 treatments in 356 attacks, were used for final statistical analysis (treatment considered as relevant to a new attack if at least 6 hours elapsed since previous treatment). Complete reporting was obtained for 70% of activations for P200, P150, and P100 programs, 58% of activations for P50, and 28% of placebo activations. No adverse events related to the device and no side effects were reported.
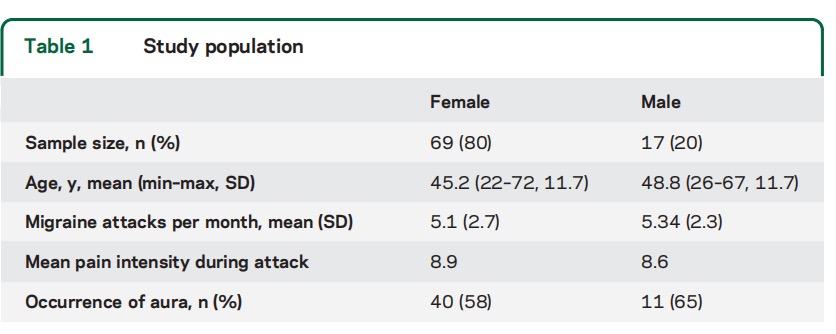
Pain reduction. Percent responders for 50% pain reduction was 46% for the strongest stimuli P200, as opposed to 26% for sham. For the next strongest P150 stimulus, percent responders was 48% (table 2). When taking all active stimulation protocols together, i.e., considering best response per individual, 64% of the patients had more than 50% pain reduction in more than half of their treated attacks and are considered responders to the evaluated treatment. This is higher than the 26% response rate to placebo activations (p = 0.005). Relative pain reduction for the active stimuli ranged between 16% and 26%, while for the placebo stimulation the reduction was only 2% (table 2). Mean pain level at device activation point was 4.6. Overall ANOVA-based effect was significant (p=0.031), with significant effect in post hoc analyses for the P150 protocol.
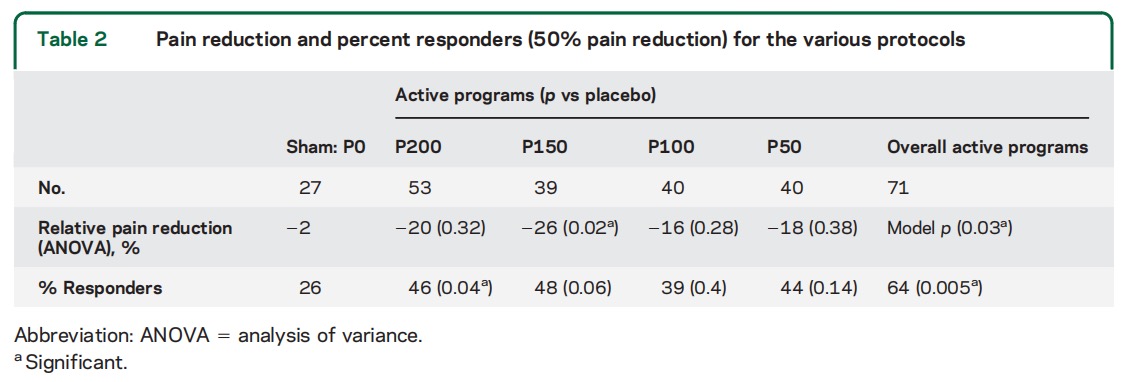
In terms of change in pain grades when calculation is based on start point of moderate and severe pain, reduction from these levels to mild or no pain was reported by 58% of the participants in response to the strongest stimulation program (P200, widest pulse), as opposed to 24% for placebo (table 3), resulting in NNT of 2.9. In the course of the study, 76% of participants who provided feedback on at least 1 active program treatment reported pain grade reduction in response to at least 1 type of active stimulation in majority of activations (significant at 0.005 level vs placebo). Pain free outcome occurred in more than 50% of activations for 30% of participants when the strongest program was activated, as opposed to 6% for placebo, resulting in NNT of 4.2. Considering also treatments with mild pain at baseline, pain-free outcome occurred in more than 50% of activations for 44% (24/54) of participants when the strongest program was activated, as opposed to 25% (6/24) for placebo.
Timing effect. Participants were instructed to activate the device as early as possible in the migraine attack. Pain reduction was highest when applied within the first 20 minutes from attack onset as opposed to when treatment was delayed and applied 20–180 minutes after attack onset—mean pain relief 46.8% vs 24.9% (p 5 0.02). Notably, no complete pain relief occurred for treatments started later than 60 minutes from pain onset. For placebo, no effect of time on pain reduction was found.
Treatment perception. Treatment perception of the 3 active programs was rated by participants as follows: painful 11%, unpleasant 28%, pleasant 58%, very pleasant 4%. For placebo, respectively: 1%, 13%, 61%, 25%.
In the end-of-trial questioning, participants indicated the following: (1) reduction in amount of migraine medications during study period—mean questionnaire score was 1.5, where 1 means same amount, 2 means less; (2) overall burden of treatment was considered very low—mean score is 2.5 (between neutral and not at all); and (3) the device and application were found easy to use by the majority of study participants—mean questionnaire score is 3.85 (between easy and very easy).
DISCUSSION
In the end-of-trial questioning, participants indicated the following: (1) reduction in amount of migraine medications during study period—mean questionnaire score was 1.5, where 1 means same amount, 2 means less; (2) overall burden of treatment was considered very low—mean score is 2.5 (between neutral and not at all); and (3) the device and application were found easy to use by the majority of study participants—mean questionnaire score is 3.85 (between easy and very easy).
For a stimulation given remotely from clinical pain site to work, a central inhibitory effect must be activated. Diffuse noxious inhibitory control, and its human counterpart, CPM, represent exertion of pain reduction by a remote conditioning noxious stimuli. This study peruses this mechanism in clinical practice, using a well-felt but non-noxious conditioning stimulus, such as heat at below pain threshold, for CPM induction in healthy participants. Further, we have recently reported induction of pain inhibition by large body area innocuous compression. Removal of ongoing spinal upgoing traffic by high epidural anesthesia was sufficient to cause increased pain perception in the face. The remoteness of our stimulation from the pain site precludes the option of classical gate control mechanism, since the latter works within segmental limits.
Overall, reported pain reduction by CPM is not large, ranging around 30%. The effect of conditioning by electrical stimuli in painful diabetic neuropathy is reported to be 25%–35%. Migraine provides an ideal model for interventions that can exert only a mild pain reduction, since it is a cyclical pain syndrome, where each attack starts from practically no pain, and then develops along several hours. It is migraine patients’ common wisdom that low dose of medication taken immediately upon attack onset is more effective than higher dose taken during a fully developed attack. The initial stage of an attack thus provides a window for our intervention, and our assumption was that the sooner the use, the better the effect. The device could be discreetly put under sleeves, and activated via a smartphone, no wires involved, thus giving the patient the freedom to use it under any social/work circumstances. Further, a nonpharmacologic device seems to be much preferred by many migraineurs, who expressed this approach in the recruitment interview. Our results show a clear advantage of the stimulation protocols over the sham one. Further, a certain stimulus response effect can be seen, with the more intense treatments giving higher effect than the less intense ones.
Our results in terms of NNT lay in the range of 2.9 (P200) to 6.25 (P100) and are comparable to those reported for a range of neuropathic pain treatments (2.0–6.8) and for triptans in migraine treatment (3.61–5.97).19 They suggest that the individual selection of the stimulus properties yields better results than a uniform stimulus for all. The same observations are true when pain-free response is considered.
Interestingly, our extent of pain relief is almost identical to that reported for triptans: 59% transition from severe or moderate to mild or no pain for triptans parallels the 58% reported here; 29% and 30% are the respective numbers for transition to no pain. A possible interpretation that this is the ceiling of the analgesic effect cannot be accepted, since injected triptans achieve better results. It might be that triptans activate the same descending tract pathways, a common final pathway of the 2 methods. A study with dual treatment could shed light on this supposition.
Compared to occipital nerve stimulation (ONS),we suggest an easy to apply, noninvasive stimulation. ONS seems more relevant to therapy-resistant cases. Some evidence favoring another invasive procedure, sphenopalatine ganglion stimulation, in episodic migraine has been raised, and further evidence is awaited. Of the noninvasive stimulation methods, fore head skin stimulation (Cefaly) is reported to be effective as a preventive mode. From the usability standpoint, it seems that its use during migraine episodes would have some practical disadvantage due to its high visibility. Noninvasive vagal nerve stimulation has been shown effective in an open-label study of episodic migraine and in prevention of chronic migraine. It requires application of the device to the neck, again, with some visibility, which might not always be desirable. Single transcranial magnetic stimulation has been shown effective for migraine with aura, relevant only to a minority of migraineurs.
Limitations. The lower rates of completion of the 20 minutes of stimulus in the placebo stimuli might indicate that some participants might have identified those stimuli as nonactive, and stopped them prematurely, since they realized no pain relief was to be expected. Maintaining blinding in studies involving neurostimulation treatments is a known challenge. Although our observation suggests that blinding was not complete, it is likely that this fact did not lead to falsely improved results; on the contrary, had those incomplete stimuli periods been completed, it is most likely that sham effectiveness results would have been lower than currently reported, making the results even more distinct. Another possible study limitation is that no information on major demographic features, beyond age and sex, was collected.
This clinical application was developed based the conditioned pain modulation concept in pain alleviation. Although we did not provide imaging- or neurophysiologic-based proof that this was the underlying mechanism, it is likely that this is the case. Considering the favorable combination of high efficacy, convenience, and excellent safety profile of this treatment—with literally no side effects—this study provides a strong basis towards widespread clinical use of remote electrical stimulation as a tool for alleviation of migraine attacks.
